Chirality of the cytoskeleton in the origins of cellular asymmetry
- PMID: 27821520
- PMCID: PMC5104507
- DOI: 10.1098/rstb.2015.0408
Chirality of the cytoskeleton in the origins of cellular asymmetry
Abstract
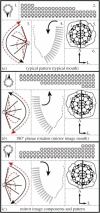
Self-assembly of two important components of the cytoskeleton of eukaryotic cells, actin microfilaments and microtubules (MTs) results in polar filaments of one chirality. As is true for bacterial flagella, in actin microfilaments, screw direction is important for assembly processes and motility. For MTs, polar orientation within the cell is paramount. The alignment of these elements in the cell cytoplasm gives rise to emergent properties, including the potential for cell differentiation and specialization. Complex MTs with a characteristic chirality are found in basal bodies and centrioles; this chirality is preserved in cilia. In motile cilia, it is reflected in the direction of the effective stroke. The positioning of the basal body or cilia on the cell surface depends on polarity proteins. In evolution, survival depends on global polarity information relayed to the cell in part by orientation of the MT and actin filament cytoskeletons and the chirality of the basal body to determine left and right coordinates within a defined anterior-posterior cell and tissue axis.This article is part of the themed issue 'Provocative questions in left-right asymmetry'.
Keywords: actin filament; asymmetry; bacterial flagellum; basal body; cilia; microtubules.
© 2016 The Author(s).
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

