Cell chirality: its origin and roles in left-right asymmetric development
- PMID: 27821533
- PMCID: PMC5104503
- DOI: 10.1098/rstb.2015.0403
Cell chirality: its origin and roles in left-right asymmetric development
Abstract
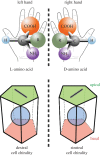
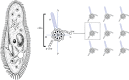
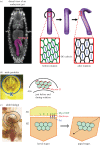
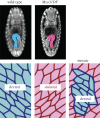
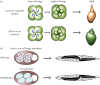
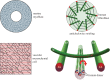
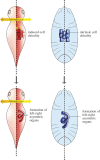
An item is chiral if it cannot be superimposed on its mirror image. Most biological molecules are chiral. The homochirality of amino acids ensures that proteins are chiral, which is essential for their functions. Chirality also occurs at the whole-cell level, which was first studied mostly in ciliates, single-celled protozoans. Ciliates show chirality in their cortical structures, which is not determined by genetics, but by 'cortical inheritance'. These studies suggested that molecular chirality directs whole-cell chirality. Intriguingly, chirality in cellular structures and functions is also found in metazoans. In Drosophila, intrinsic cell chirality is observed in various left-right (LR) asymmetric tissues, and appears to be responsible for their LR asymmetric morphogenesis. In other invertebrates, such as snails and Caenorhabditis elegans, blastomere chirality is responsible for subsequent LR asymmetric development. Various cultured cells of vertebrates also show intrinsic chirality in their cellular behaviours and intracellular structural dynamics. Thus, cell chirality may be a general property of eukaryotic cells. In Drosophila, cell chirality drives the LR asymmetric development of individual organs, without establishing the LR axis of the whole embryo. Considering that organ-intrinsic LR asymmetry is also reported in vertebrates, this mechanism may contribute to LR asymmetric development across phyla.This article is part of the themed issue 'Provocative questions in left-right asymmetry'.
Keywords: cell chirality; cortical inheritance; f actin; left–right asymmetry; myosin I.
© 2016 The Authors.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

