Recombinase Polymerase Amplification Compared to Real-Time Polymerase Chain Reaction Test for the Detection of Fasciola hepatica in Human Stool
- PMID: 27821691
- PMCID: PMC5303034
- DOI: 10.4269/ajtmh.16-0601
Recombinase Polymerase Amplification Compared to Real-Time Polymerase Chain Reaction Test for the Detection of Fasciola hepatica in Human Stool
Abstract
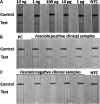
Fasciola hepatica is the most widely distributed trematode infection in the world. Control efforts may be hindered by the lack of diagnostic capacity especially in remote endemic areas. Polymerase chain reaction (PCR)-based methods offer high sensitivity and specificity but require expensive technology. However, the recombinase polymerase amplification (RPA) is an efficient isothermal method that eliminates the need for a thermal cycler and has a high deployment potential to resource-limited settings. We report on the characterization of RPA and PCR tests to detect Fasciola infection in clinical stool samples with low egg burdens. The sensitivity of the RPA and PCR were 87% and 66%, respectively. Both tests were 100% specific showing no cross-reactivity with trematode, cestode, or nematode parasites. In addition, RPA and PCR were able to detect 47% and 26% of infections not detected by microscopy, respectively. The RPA adapted to a lateral flow platform was more sensitive than gel-based detection of the reaction products. In conclusion, the Fasciola RPA is a highly sensitive and specific test to diagnose chronic infection using stool samples. The Fasciola RPA lateral flow has the potential for deployment to endemic areas after further characterization.
© The American Society of Tropical Medicine and Hygiene.
Figures


References
-
- Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–221. - PubMed
-
- Fentie T, Erqou S, Gedefaw M, Desta A. Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, northwest Ethiopia. Trans R Soc Trop Med Hyg. 2013;107:480–486. - PubMed
-
- Nguyen TGT. Zoonotic Fasciolosis in Vietnam: Molecular Identification and Geographical Distribution. 2012. http://www.vpi.ugent.be/page13/files/giang-thanh-nguyen-thi-2.pdf Available at. Accessed March 25, 2015.
-
- Moghaddam AS, Massoud J, Mahmoodi M, Mahvi AH, Periago MV, Artigas P, Fuentes MV, Bargues MD, Mas-Coma S. Human and animal fascioliasis in Mazandaran province, northern Iran. Parasitol Res. 2004;94:61–69. - PubMed
-
- Steinmann P, Usubalieva J, Imanalieva C, Minbaeva G, Stefiuk K, Jeandron A, Utzinger J. Rapid appraisal of human intestinal helminth infections among schoolchildren in Osh oblast, Kyrgyzstan. Acta Trop. 2010;116:178–184. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

