Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands
- PMID: 27821701
- PMCID: PMC5098549
- DOI: 10.1136/bmj.i5789
Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands
Abstract
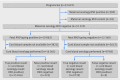
Objective: To determine the accuracy of non-invasive fetal testing for the RHD gene in week 27 of pregnancy as part of an antenatal screening programme to restrict anti-D immunoglobulin use to women carrying a child positive for RHD DESIGN: Prospectively monitoring of fetal RHD testing accuracy compared with serological cord blood typing on introduction of the test. Fetal RHD testing was performed with a duplex real time quantitative polymerase chain reaction, with cell-free fetal DNA isolated from 1 mL of maternal plasma The study period was between 4 July 2011 and 7 October 2012. The proportion of women participating in screening was determined.
Setting: Nationwide screening programme, the Netherlands. Tests are performed in a centralised setting.
Participants: 25 789 RhD negative pregnant women.
Main outcome measures: Sensitivity, specificity, false negative rate, and false positive rate of fetal RHD testing compared with serological cord blood typing; proportion of technical failures; and compliance to the screening programme.
Results: A fetal RHD test result and serological cord blood result were available for 25 789 pregnancies. Sensitivity for detection of fetal RHD was 99.94% (95% confidence interval 99.89% to 99.97%) and specificity was 97.74% (97.43% to 98.02%). Nine false negative results for fetal RHD testing were registered (0.03%, 95% confidence interval 0.01% to 0.06%). In two cases these were due to technical failures. False positive fetal RHD testing results were registered for 225 samples (0.87%, 0.76% to 0.99%). Weak RhD expression was shown in 22 of these cases, justifying anti-D immunoglobulin use. The negative and positive predictive values were 99.91% (95% confidence interval 99.82% to 99.95%) and 98.60% (98.40% to 98.77%), respectively. More than 98% of the women participated in the screening programme.
Conclusions: Fetal RHD testing in week 27 of pregnancy as part of a national antenatal screening programme is highly reliable and can be used to target both antenatal and postnatal anti-D immunoglobulin use.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
All authors completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
PURL: A new protocol for RhD-negative pregnant women?J Fam Pract. 2018 May;67(5):306;308;319. J Fam Pract. 2018. PMID: 29726855 Free PMC article.
References
-
- Urbaniak SJ, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev 2000;14:44-61. 10.1054/blre.1999.0123 pmid:10805260. - DOI - PubMed
-
- de Haas M, Thurik FF, Koelewijn JM, van der Schoot CE. Haemolytic disease of the fetus and newborn. Vox Sang 2015;109:99-113. 10.1111/vox.12265 pmid:25899660. - DOI - PubMed
-
- Crowther C, Middleton P. Anti-D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 2000;(2):CD000021.pmid:10796089. - PMC - PubMed
-
- Koelewijn JM, de Haas M, Vrijkotte TG, Bonsel GJ, van der Schoot CE. One single dose of 200 microg of antenatal RhIG halves the risk of anti-D immunization and hemolytic disease of the fetus and newborn in the next pregnancy. Transfusion 2008;48:1721-9. 10.1111/j.1537-2995.2008.01742.x pmid:18507749. - DOI - PubMed
-
- Pilgrim H, Lloyd-Jones M, Rees A. Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation. Health Technol Assess 2009;13(10):iii,ix-xi,1-103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical