Proceedings of the 2016 National Toxicology Program Satellite Symposium
- PMID: 27821709
- PMCID: PMC5225145
- DOI: 10.1177/0192623316672074
Proceedings of the 2016 National Toxicology Program Satellite Symposium
Abstract
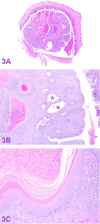
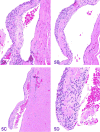
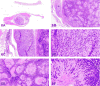
The 2016 annual National Toxicology Program Satellite Symposium, entitled "Pathology Potpourri" was held in San Diego, CA, at the Society of Toxicologic Pathology's (STP) 35th annual meeting. The goal of this symposium was to present and discuss challenging diagnostic pathology and/or nomenclature issues. This article presents summaries of the speakers' talks, along with select images that were used by the audience for voting and discussion. Some lesions and topics covered during the symposium included malignant glioma and histiocytic sarcoma in the rodent brain; a new statistical method designed for histopathology data evaluation; uterine stromal/glandular polyp in a rat; malignant plasma cell tumor in a mouse brain; Schwann cell proliferative lesions in rat hearts; axillary schwannoma in a cat; necrosis and granulomatous inflammation in a rat brain; adenoma/carcinoma in a rat adrenal gland; hepatocyte maturation defect and liver/spleen hematopoietic defects in an embryonic mouse; distinguishing malignant glioma, malignant mixed glioma, and malignant oligodendroglioma in the rat; comparison of mammary gland whole mounts and histopathology from mice; and discussion of the International Harmonization of Nomenclature and Diagnostic Criteria collaborations.
Keywords: INHAND; NTP Satellite Symposium; Rao-Scott Cochran-Armitage by Slices; abnormal mouse fetal development; adrenal gland carcinoma; brain plasma cell tumor; endometrial polyp; glioblastoma multiforme; malignant glioma; mammary gland whole mount; schwannoma.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures























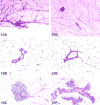
References
-
- Adams ET, Auerbach S, Blackshear PE, Bradley A, Gruebbel MM, Little PB, Malarkey D, Maronpot R, McKay JS, Miller RA, Moore RR, Morrison JP, Nyska A, Ramot Y, Rao D, Suttie A, Wells MY, Willson GA, Elmore SA. Proceedings of the 2010 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2011;39:240–66. - PMC - PubMed
-
- Alison RH, Elwell MR, Jokinen MP, Dittrich KL, Boorman GA. Morphology and classification of 96 primary cardiac neoplasms in Fischer 344 rats. Vet Pathol. 1987;24:488–94. - PubMed
-
- Bach U, Hailey JR, Hill GD, Kaufmann W, Latimer KS, Malarkey DE, Maronpot RM, Miller RA, Moore RR, Morrison JP, Nolte T, Rinke M, Rittinghausen S, Suttie AW, Travlos GS, Vahle JL, Willson GA, Elmore SA. Proceedings of the 2009 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2010;38:9–36. - PMC - PubMed
-
- Bolon B. The case for developmental pathology and developmental pathologists. CRC Press; India: 2015. pp. 3–9.pp. 1–430.
-
- Bolon B, Carreira V. Pathology of the developing mouse from conception to weaning. In: Bolon B, editor. Pathology of the developing mouse: a systematic approach. CRC Press; Boca Raton: 2015. pp. 293–253.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

