Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks
- PMID: 27821730
- PMCID: PMC5127353
- DOI: 10.1073/pnas.1608767113
Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks
Erratum in
-
Correction for Artemenko et al., Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks.Proc Natl Acad Sci U S A. 2018 Jul 10;115(28):E6669. doi: 10.1073/pnas.1809926115. Epub 2018 Jun 25. Proc Natl Acad Sci U S A. 2018. PMID: 29941574 Free PMC article. No abstract available.
Abstract
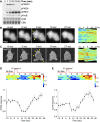
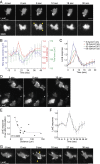
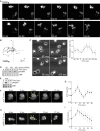
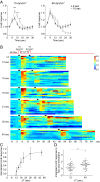
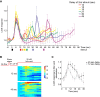
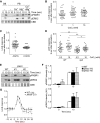
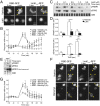
Signal transduction pathways activated by chemoattractants have been extensively studied, but little is known about the events mediating responses to mechanical stimuli. We discovered that acute mechanical perturbation of cells triggered transient activation of all tested components of the chemotactic signal transduction network, as well as actin polymerization. Similarly to chemoattractants, the shear flow-induced signal transduction events displayed features of excitability, including the ability to mount a full response irrespective of the length of the stimulation and a refractory period that is shared with that generated by chemoattractants. Loss of G protein subunits, inhibition of multiple signal transduction events, or disruption of calcium signaling attenuated the response to acute mechanical stimulation. Unlike the response to chemoattractants, an intact actin cytoskeleton was essential for reacting to mechanical perturbation. These results taken together suggest that chemotactic and mechanical stimuli trigger activation of a common signal transduction network that integrates external cues to regulate cytoskeletal activity and drive cell migration.
Keywords: biochemical excitability; biomechanics; inflammation; motility; shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Cortese B, Palamà IE, D’Amone S, Gigli G. Influence of electrotaxis on cell behaviour. Integr Biol (Camb) 2014;6(9):817–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

