A Snapshot on the On-Label and Off-Label Use of the Interleukin-1 Inhibitors in Italy among Rheumatologists and Pediatric Rheumatologists: A Nationwide Multi-Center Retrospective Observational Study
- PMID: 27822185
- PMCID: PMC5076463
- DOI: 10.3389/fphar.2016.00380
A Snapshot on the On-Label and Off-Label Use of the Interleukin-1 Inhibitors in Italy among Rheumatologists and Pediatric Rheumatologists: A Nationwide Multi-Center Retrospective Observational Study
Abstract
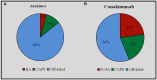
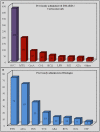
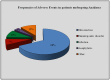
Background: Interleukin (IL)-1 inhibitors have been suggested as possible therapeutic options in a large number of old and new clinical entities characterized by an IL-1 driven pathogenesis. Objectives: To perform a nationwide snapshot of the on-label and off-label use of anakinra (ANA) and canakinumab (CAN) for different conditions both in children and adults. Methods: We retrospectively collected demographic, clinical, and therapeutic data from both adult and pediatric patients treated with IL-1 inhibitors from January 2008 to July 2016. Results: Five hundred and twenty-six treatment courses given to 475 patients (195 males, 280 females; 111 children and 364 adults) were evaluated. ANA was administered in 421 (80.04%) courses, CAN in 105 (19.96%). Sixty-two (32.1%) patients had been treated with both agents. IL-1 inhibitors were employed in 38 different indications (37 with ANA, 16 with CAN). Off-label use was more frequent for ANA than CAN (p < 0.0001). ANA was employed as first-line biologic approach in 323 (76.7%) cases, while CAN in 37 cases (35.2%). IL-1 inhibitors were associated with corticosteroids in 285 (54.18%) courses and disease modifying anti-rheumatic drugs (DMARDs) in 156 (29.65%). ANA dosage ranged from 30 to 200 mg/day (or 1.0-2.0 mg/kg/day) among adults and 2-4 mg/kg/day among children; regarding CAN, the most frequently used posologies were 150mg every 8 weeks, 150mg every 4 weeks and 150mg every 6 weeks. The frequency of failure was higher among patients treated with ANA at a dosage of 100 mg/day than those treated with 2 mg/kg/day (p = 0.03). Seventy-six patients (14.4%) reported an adverse event (AE) and 10 (1.9%) a severe AE. AEs occurred more frequently after the age of 65 compared to both children and patients aged between 16 and 65 (p = 0.003 and p = 0.03, respectively). Conclusions: IL-1 inhibitors are mostly used off-label, especially ANA, during adulthood. The high frequency of good clinical responses suggests that IL-1 inhibitors are used with awareness of pathogenetic mechanisms; adult healthcare physicians generally employ standard dosages, while pediatricians are more prone in using a weight-based posology. Dose adjustments and switching between different agents showed to be effective treatment strategies. Our data confirm the good safety profile of IL-1 inhibitors.
Keywords: anakinra; autoinflammatory disorders; canakinumab; interleukin (IL)-1; treatment.
Figures





References
-
- Abbate A., Van Tassell B. W., Biondi-Zoccai G., Kontos M. C., Grizzard J. D., Spillman D. W., et al. . (2013). Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 111, 1394–1400. 10.1016/j.amjcard.2013.01.287 - DOI - PMC - PubMed
-
- Alten R., Gomez-Reino J., Durez P., Beaulieu A., Sebba A., Krammer G., et al. . (2011). Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase, I. I., dose-finding study. BMC Musculoskelet. Disord. 12:153. 10.1186/1471-2474-12-153 - DOI - PMC - PubMed
-
- Annicchiarico G., Lopalco G., Morgese M. G., Cantarini L., Iannone F. (2016). Canakinumab in recessive dystrophic epidermolysis bullosa: a novel unexpected weapon for non-healing wounds? Clin. Exp. Rheumatol. 34, 961–962. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

