Micro- and Macrostructured PLGA/Gelatin Scaffolds Promote Early Cardiogenic Commitment of Human Mesenchymal Stem Cells In Vitro
- PMID: 27822229
- PMCID: PMC5086396
- DOI: 10.1155/2016/7176154
Micro- and Macrostructured PLGA/Gelatin Scaffolds Promote Early Cardiogenic Commitment of Human Mesenchymal Stem Cells In Vitro
Abstract
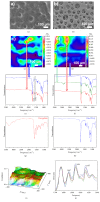
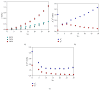
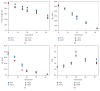
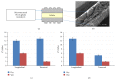
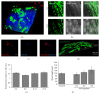
The biomaterial scaffold plays a key role in most tissue engineering strategies. Its surface properties, micropatterning, degradation, and mechanical features affect not only the generation of the tissue construct in vitro, but also its in vivo functionality. The area of myocardial tissue engineering still faces significant difficulties and challenges in the design of bioactive scaffolds, which allow composition variation to accommodate divergence in the evolving myocardial structure. Here we aimed at verifying if a microstructured bioartificial scaffold alone can provoke an effect on stem cell behavior. To this purpose, we fabricated microstructured bioartificial polymeric constructs made of PLGA/gelatin mimicking anisotropic structure and mechanical properties of the myocardium. We found that PLGA/gelatin scaffolds promoted adhesion, elongation, ordered disposition, and early myocardial commitment of human mesenchymal stem cells suggesting that these constructs are able to crosstalk with stem cells in a precise and controlled manner. At the same time, the biomaterial degradation kinetics renders the PLGA/gelatin constructs very attractive for myocardial regeneration approaches.
Figures


References
-
- Jana S., Tefft B. J., Spoon D. B., Simari R. D. Scaffolds for tissue engineering of cardiac valves. Acta Biomaterialia. 2014;10:2877–2893. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

