Acute Axonal Degeneration Drives Development of Cognitive, Motor, and Visual Deficits after Blast-Mediated Traumatic Brain Injury in Mice
- PMID: 27822499
- PMCID: PMC5086797
- DOI: 10.1523/ENEURO.0220-16.2016
Acute Axonal Degeneration Drives Development of Cognitive, Motor, and Visual Deficits after Blast-Mediated Traumatic Brain Injury in Mice
Abstract
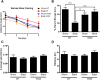
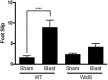
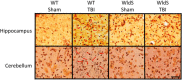
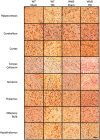
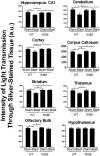
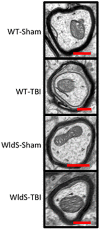
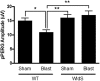
Axonal degeneration is a prominent feature of many forms of neurodegeneration, and also an early event in blast-mediated traumatic brain injury (TBI), the signature injury of soldiers in Iraq and Afghanistan. It is not known, however, whether this axonal degeneration is what drives development of subsequent neurologic deficits after the injury. The Wallerian degeneration slow strain (WldS) of mice is resistant to some forms of axonal degeneration because of a triplicated fusion gene encoding the first 70 amino acids of Ufd2a, a ubiquitin-chain assembly factor, that is linked to the complete coding sequence of nicotinamide mononucleotide adenylyltransferase 1 (NMAT1). Here, we demonstrate that resistance of WldS mice to axonal degeneration after blast-mediated TBI is associated with preserved function in hippocampal-dependent spatial memory, cerebellar-dependent motor balance, and retinal and optic nerve-dependent visual function. Thus, early axonal degeneration is likely a critical driver of subsequent neurobehavioral complications of blast-mediated TBI. Future therapeutic strategies targeted specifically at mitigating axonal degeneration may provide a uniquely beneficial approach to treating patients suffering from the effects of blast-mediated TBI.
Keywords: WldS mouse; axonal degeneration; neurodegeneration; nicotinamide adenine dinucleotide; traumatic brain injury.
Conflict of interest statement
Authors report no conflict of interest.
Figures
References
-
- Baldock RA, Poole I (1993) Video camera calibration for optical densitometry. J Microsc Oct. 172(Pt1):49–54. - PubMed
-
- Clark RS, Vagni VA, Nathaniel PD, Jenkins LW, Dixon CE, Szabó C (2007) Local administration of the poly(ADP-ribose) polymerase inhibitor INO-1001 prevents NAD+ depletion and improves water maze performance after traumatic brain injury in mice. J Neurotrauma 24:1399–1405. 10.1089/neu.2007.0305 - DOI - PubMed
-
- Cockerham GC, Goodrich GL, Weichel ED, Orcutt JC, Rizzo JF, Bower KSA, Schuchard RA (2009) Eye and visual function in traumatic brain injury. J Rehabil Res Dev 46:811–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
