Reproducibility of 18F-fluoromisonidazole intratumour distribution in non-small cell lung cancer
- PMID: 27822900
- PMCID: PMC5099292
- DOI: 10.1186/s13550-016-0210-y
Reproducibility of 18F-fluoromisonidazole intratumour distribution in non-small cell lung cancer
Abstract
Background: Hypoxic tumours exhibit increased resistance to radiation, chemical, and immune therapies. 18F-fluoromisonidazole (FMISO) positron emission tomography (PET) is a non-invasive, quantitative imaging technique used to evaluate the presence and spatial distribution of tumour hypoxia. To facilitate the use of FMISO PET for identification of individuals likely to benefit from hypoxia-targeted treatments, we investigated the reproducibility of FMISO PET spatiotemporal intratumour distribution in patients with non-small cell lung cancer (NSCLC).
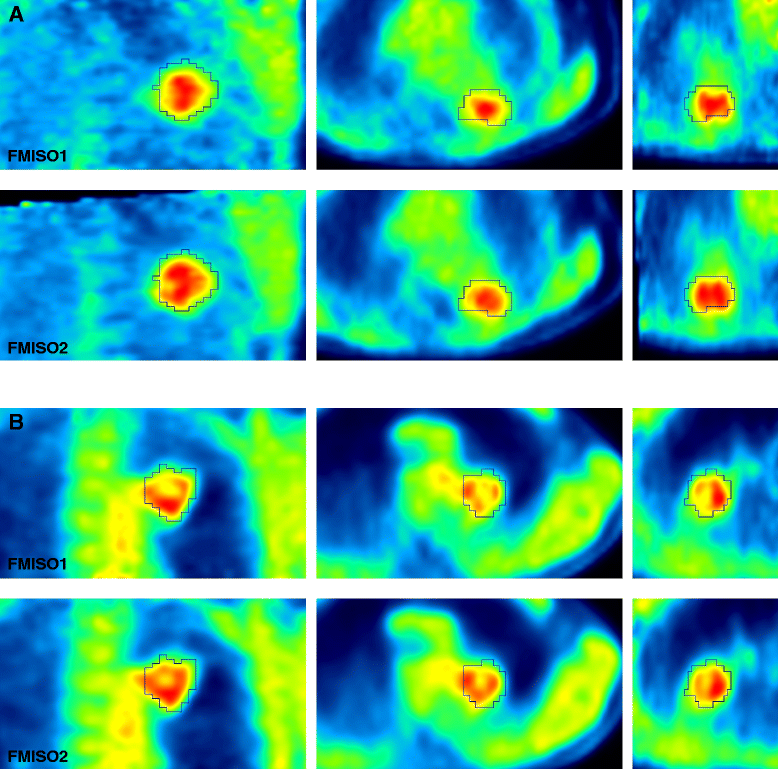
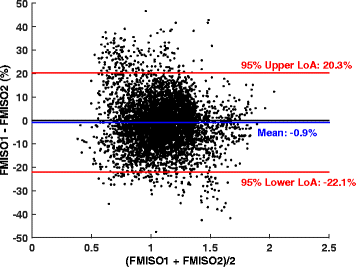
Methods: Ten patients underwent 18F-fluorodeoxyglucose (FDG) PET/CT scans, followed by two FMISO PET/CT scans 1-2 days apart. Nineteen lesions in total were segmented from co-registered FDG PET image sets. Volumes of interest were also defined on normal contralateral lung and subscapularis muscle. The Pearson correlation coefficient r was calculated for mean standardized uptake values (SUV) within investigated volumes of interest and for voxels within tumour volumes (r TV). The reproducibility of FMISO voxelwise distribution, SUV- and tumour-to-blood ratio (TBR)-derived indices was assessed using correlation and Bland-Altman analyses.
Results: The SUVmax, SUVmean, TBRmax, and TBRmean were highly correlated (r ≥ 0.87, p < 0.001) and were reproducible to within 10-15 %. The mean r TV was 0.84 ± 0.10. 77 % of voxels identified as hypoxic on one FMISO scan were confirmed as such on the other FMISO scan. Mean voxelwise differences between TBR values as calculated from pooled data including all lesions were 0.9 ± 10.8 %.
Conclusions: High reproducibility of FMISO intratumour distribution in NSCLC patients was observed, facilitating its use in determining the topology of the hypoxic tumour sub-volumes for dose escalation, in patient stratification strategies for hypoxia-targeted therapies, and in monitoring response to therapeutic interventions.
Trial registration: Current Controlled Trials NCT02016872.
Keywords: 18F-fluoromisonidazole; Hypoxia; Non-small cell lung cancer; Quantification; Reproducibility.
Figures

Similar articles
-
Timing of hypoxia PET/CT imaging after 18F-fluoromisonidazole injection in non-small cell lung cancer patients.Sci Rep. 2022 Dec 16;12(1):21746. doi: 10.1038/s41598-022-26199-7. Sci Rep. 2022. PMID: 36526815 Free PMC article.
-
Pharmacokinetic Analysis of Dynamic 18F-Fluoromisonidazole PET Data in Non-Small Cell Lung Cancer.J Nucl Med. 2017 Jun;58(6):911-919. doi: 10.2967/jnumed.116.180422. Epub 2017 Feb 23. J Nucl Med. 2017. PMID: 28232611 Free PMC article. Clinical Trial.
-
High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer.J Nucl Med. 2013 Feb;54(2):201-7. doi: 10.2967/jnumed.112.109330. Epub 2013 Jan 15. J Nucl Med. 2013. PMID: 23321456
-
[18F]Fluoromisonidazole.2005 Jul 18 [updated 2005 Aug 15]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2005 Jul 18 [updated 2005 Aug 15]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641304 Free Books & Documents. Review.
-
The Roles of Hypoxia Imaging Using 18F-Fluoromisonidazole Positron Emission Tomography in Glioma Treatment.J Clin Med. 2019 Jul 24;8(8):1088. doi: 10.3390/jcm8081088. J Clin Med. 2019. PMID: 31344848 Free PMC article. Review.
Cited by
-
[18F]FMISO PET/CT as a preoperative prognostic factor in patients with pancreatic cancer.EJNMMI Res. 2019 May 9;9(1):39. doi: 10.1186/s13550-019-0507-8. EJNMMI Res. 2019. PMID: 31073705 Free PMC article.
-
Monitoring early response to chemoradiotherapy with 18F-FMISO dynamic PET in head and neck cancer.Eur J Nucl Med Mol Imaging. 2017 Sep;44(10):1682-1691. doi: 10.1007/s00259-017-3720-6. Epub 2017 May 24. Eur J Nucl Med Mol Imaging. 2017. PMID: 28540417 Free PMC article. Clinical Trial.
-
An 18F-FDG PET/CT and Mean Lung Dose Model to Predict Early Radiation Pneumonitis in Stage III Non-Small Cell Lung Cancer Patients Treated with Chemoradiation and Immunotherapy.J Nucl Med. 2024 Apr 1;65(4):520-526. doi: 10.2967/jnumed.123.266965. J Nucl Med. 2024. PMID: 38485270 Free PMC article.
-
Oxygen-Guided Radiation Therapy.Int J Radiat Oncol Biol Phys. 2019 Mar 15;103(4):977-984. doi: 10.1016/j.ijrobp.2018.10.041. Epub 2018 Nov 8. Int J Radiat Oncol Biol Phys. 2019. PMID: 30414912 Free PMC article.
-
Multiparametric Imaging of Tumor Hypoxia and Perfusion with 18F-Fluoromisonidazole Dynamic PET in Head and Neck Cancer.J Nucl Med. 2017 Jul;58(7):1072-1080. doi: 10.2967/jnumed.116.188649. Epub 2017 Feb 9. J Nucl Med. 2017. PMID: 28183993 Free PMC article.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

