Safety and Efficacy of New Anticoagulants for the Prevention of Venous Thromboembolism After Hip and Knee Arthroplasty: A Meta-Analysis
- PMID: 27823844
- PMCID: PMC5258767
- DOI: 10.1016/j.arth.2016.09.033
Safety and Efficacy of New Anticoagulants for the Prevention of Venous Thromboembolism After Hip and Knee Arthroplasty: A Meta-Analysis
Abstract
Background: Venous thromboembolism (VTE) is a common and potentially fatal complication of arthroplasty.
Methods: We reviewed randomized trials to determine which anticoagulant has the best safety and efficacy in hip and knee arthroplasty patients. We searched PubMed, MEDLINE, and EMBASE through January 2016.
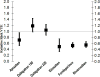
Results: Compared to enoxaparin (most commonly dosed 40 mg once daily), the relative risk (RR) of VTE was lowest for edoxaban 30 mg once daily (0.49; 95% confidence interval [CI], 0.32-0.75), fondaparinux 2.5 mg once daily (0.53; 95% CI, 0.45-0.63), and rivaroxaban 10 mg once daily (0.55; 95% CI, 0.46-0.66), and highest for dabigatran 150 mg once daily (1.19; 95% CI; 0.98-1.44). The RR of major/clinically relevant bleeding was lowest for apixaban 2.5 mg twice daily (0.84; 95% CI; 0.70-0.99) and highest for rivaroxaban (1.27; 95% CI, 1.01-1.59) and fondaparinux (1.64; 95% CI, 0.24-11.35). Fondaparinux was the only agent that was more effective than enoxaparin 30 mg twice daily (VTE RR = 0.58; 95% CI, 0.43-0.76).
Conclusion: With the possible exception of apixaban, newer anticoagulants that lower the risk of postoperative VTE increase bleeding.
Keywords: anticoagulant; arthroplasty; bleed; deep vein thrombosis; meta-analysis; thromboembolism.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
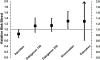
References
-
- Witt DM, Nutescu EA, Haines ST. Chapter 26. Venous Thromboembolism. In: Talbert RL, DiPiro JT, Matzke GR, Posey LM, Wells BG, Yee GC, editors. Pharmacotherapy: A Pathophysiologic Approach. 8th. New York: McGraw-Hill; 2011.
-
- Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW, Jr, American College of Chest Physicians Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e278S–325S. - PMC - PubMed
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. - PubMed
-
- Committee for Proprietary Medicinal Products. Points to Consider on Clinical Investigation of Medicinal Products for Prophylaxis of Intra and Post-Operative Venous Thromboembolic Risk. London: The European Agency for the Evaluation of Medicinal Products; 2007. (Guideline no. CPMP/EWP/707/98 Rev.1 corr.).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

