Trehalose prevents aggregation of exosomes and cryodamage
- PMID: 27824088
- PMCID: PMC5099918
- DOI: 10.1038/srep36162
Trehalose prevents aggregation of exosomes and cryodamage
Abstract
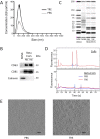
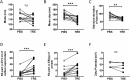
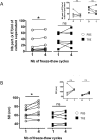
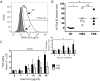
Exosomes are important mediators in intercellular communication. Released by many cell types, they transport proteins, lipids, and nucleic acids to distant recipient cells and contribute to important physiopathological processes. Standard current exosome isolation methods based on differential centrifugation protocols tend to induce aggregation of particles in highly concentrated suspensions and freezing of exosomes can induce damage and inconsistent biological activity. Trehalose is a natural, non-toxic sugar widely used as a protein stabilizer and cryoprotectant by the food and drug industry. Here we report that addition of 25 mM trehalose to pancreatic beta-cell exosome-like vesicle isolation and storage buffer narrows the particle size distribution and increases the number of individual particles per microgram of protein. Repeated freeze-thaw cycles induce an increase in particle concentration and in the width of the size distribution for exosome-like vesicles stored in PBS, but not in PBS 25 mM trehalose. No signs of lysis or incomplete vesicles were observed by cryo-electron tomography in PBS and trehalose samples. In macrophage immune assays, beta-cell extracellular vesicles in trehalose show consistently higher TNF-alpha cytokine secretion stimulation indexes suggesting improved preservation of biological activity. The addition of trehalose might be an attractive means to standardize experiments in the field of exosome research and downstream applications.
Figures
References
-
- Ramachandra L. et al.. Mycobacterium tuberculosis Synergizes with ATP To Induce Release of Microvesicles and Exosomes Containing Major Histocompatibility Complex Class II Molecules Capable of Antigen Presentation. Infection and Immunity 78, 5116–5125, doi: 10.1128/iai.01089-09 (2010). - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

