Bifunctional Anti-Non-Amyloid Component α-Synuclein Nanobodies Are Protective In Situ
- PMID: 27824888
- PMCID: PMC5100967
- DOI: 10.1371/journal.pone.0165964
Bifunctional Anti-Non-Amyloid Component α-Synuclein Nanobodies Are Protective In Situ
Abstract
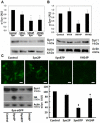
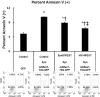
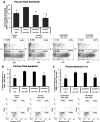
Misfolding, abnormal accumulation, and secretion of α-Synuclein (α-Syn) are closely associated with synucleinopathies, including Parkinson's disease (PD). VH14 is a human single domain intrabody selected against the non-amyloid component (NAC) hydrophobic interaction region of α-Syn, which is critical for initial aggregation. Using neuronal cell lines, we show that as a bifunctional nanobody fused to a proteasome targeting signal, VH14PEST can counteract heterologous proteostatic effects of mutant α-Syn on mutant huntingtin Exon1 and protect against α-Syn toxicity using propidium iodide or Annexin V readouts. We compared this anti-NAC candidate to NbSyn87, which binds to the C-terminus of α-Syn. NbSyn87PEST degrades α-Syn as well or better than VH14PEST. However, while both candidates reduced toxicity, VH14PEST appears more effective in both proteostatic stress and toxicity assays. These results show that the approach of reducing intracellular monomeric targets with novel antibody engineering technology should allow in vivo modulation of proteostatic pathologies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51(2 Suppl 2):S2–9. . - PubMed
-
- Forno LS. Neuropathology of Parkinson's disease. Journal of neuropathology and experimental neurology. 1996;55(3):259–72. . - PubMed
-
- Mezey E, Dehejia AM, Harta G, Tresser N, Suchy SF, Nussbaum RL, et al. Alpha synuclein is present in Lewy bodies in sporadic Parkinson's disease. Molecular psychiatry. 1998;3(6):493–9. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

