High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy
- PMID: 27825100
- PMCID: PMC5161522
- DOI: 10.1096/fj.201600293R
High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy
Abstract
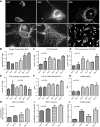
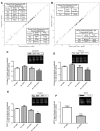
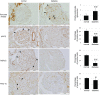
Podocytes play a key role in diabetic nephropathy pathogenesis, but alteration of their metabolism remains unknown in human kidney. By using a conditionally differentiating human podocyte cell line, we addressed the functional and molecular changes in podocyte energetics during in vitro development or under high glucose conditions. In 5 mM glucose medium, we observed a stepwise activation of oxidative metabolism during cell differentiation that was characterized by peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α)-dependent stimulation of mitochondrial biogenesis and function, with concomitant reduction of the glycolytic enzyme content. Conversely, when podocytes were cultured in high glucose (20 mM), stepwise oxidative phosphorylation biogenesis was aborted, and a glycolytic switch occurred, with consecutive lactic acidosis. Expression of the master regulators of oxidative metabolism transcription factor A mitochondrial, PGC-1α, AMPK, and serine-threonine liver kinase B1 was altered by high glucose, as well as their downstream signaling networks. Focused transcriptomics revealed that myocyte-specific enhancer factor 2C (MEF2C) and myogenic factor 5 (MYF5) expression was inhibited by high glucose levels, and endoribonuclease-prepared small interfering RNA-mediated combined inhibition of those transcription factors phenocopied the glycolytic shift that was observed in high glucose conditions. Accordingly, a reduced expression of MEF2C, MYF5, and PGC-1α was found in kidney tissue sections that were obtained from patients with diabetic nephropathy. These findings obtained in human samples demonstrate that MEF2C-MYF5-dependent bioenergetic dedifferentiation occurs in podocytes that are confronted with a high-glucose milieu.-Imasawa, T., Obre, E., Bellance, N., Lavie, J., Imasawa, T., Rigothier, C., Delmas, Y., Combe, C., Lacombe, D., Benard, G., Claverol, S., Bonneu, M., Rossignol, R. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy.
Keywords: MEF2C; human kidney; mitochondria.
© The Author(s).
Figures


References
-
- Dronavalli S., Duka I., Bakris G. L. (2008) The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 4, 444–452 - PubMed
-
- Gassler N., Elger M., Kränzlin B., Kriz W., Gretz N., Hähnel B., Hosser H., Hartmann I. (2001) Podocyte injury underlies the progression of focal segmental glomerulosclerosis in the fa/fa Zucker rat. Kidney Int. 60, 106–116 - PubMed
-
- Hara M., Yamagata K., Tomino Y., Saito A., Hirayama Y., Ogasawara S., Kurosawa H., Sekine S., Yan K. (2012) Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 55, 2913–2919 - PMC - PubMed
-
- Li J. J., Kwak S. J., Jung D. S., Kim J. J., Yoo T. H., Ryu D. R., Han S. H., Choi H. Y., Lee J. E., Moon S. J., Kim D. K., Han D. S., Kang S. W. (2007) Podocyte biology in diabetic nephropathy. Kidney Int. Suppl. 72(106), S36–S42 - PubMed
-
- Ihalmo P., Wessman M., Kaunisto M. A., Kilpikari R., Parkkonen M., Forsblom C., Holthöfer H., Groop P. H.; FinnDiane Study Group (2008) Association analysis of podocyte slit diaphragm genes as candidates for diabetic nephropathy. Diabetologia 51, 86–90 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

