Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model
- PMID: 27825113
- PMCID: PMC5347754
- DOI: 10.18632/oncotarget.13068
Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model
Abstract
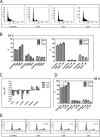
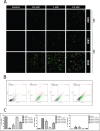
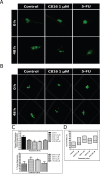
The marine environment constitutes an extraordinary resource for the discovery of new therapeutic agents. In the present manuscript we studied the effect of 3 different sponge derived guanidine alkaloids, crambescidine-816, -830, and -800. We show that these compounds strongly inhibit tumor cell proliferation by down-regulating cyclin-dependent kinases 2/6 and cyclins D/A expression while up-regulating the cell cyclin-dependent kinase inhibitors -2A, -2D and -1A. We also show that these guanidine compounds disrupt tumor cell adhesion and cytoskeletal integrity promoting the activation of the intrinsic apoptotic signaling, resulting in loss of mitochondrial membrane potential and concomitant caspase-3 cleavage and activation. The crambescidin 816 anti-tumor effect was fnally assayed in a zebrafish xenotransplantation model confirming its potent antitumor activity against colorectal carcinoma in vivo.Considering these results crambescidins could represent promising natural anticancer agents and therapeutic tools.
Keywords: apoptosis; cancer treatment; cell cycle inhibition; crambescidins; zebrafish xenograft model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Reviews Drug Discovery. 2009;8:69–85. - PubMed
-
- Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends in pharmacological sciences. 2010;31:255–265. - PubMed
-
- Berlinck R, Braekman JC, Daloze D, Bruno I, Riccio R, Ferri S, Spampinato S, Speroni E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. Journal of natural products. 1993;56:1007–1015. - PubMed
-
- Berlinck R, Braekman JC, Daloze D, Hallenga K, Ottinger R, Bruno I, Riccio R. Two new guanidine alkaloids from the mediterranean sponge crambe crambe. Tetrahedron letters. 1990;31:6531–6534.
-
- Jares-Erijman EA, Sakai R, Rinehart KL. Crambescidins: new antiviral and cytotoxic compounds from the sponge Crambe crambe. The Journal of Organic Chemistry. 1991;56:5712–5715.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

