Growth hormone is increased in the lungs and enhances experimental lung metastasis of melanoma in DJ-1 KO mice
- PMID: 27825319
- PMCID: PMC5101681
- DOI: 10.1186/s12885-016-2898-5
Growth hormone is increased in the lungs and enhances experimental lung metastasis of melanoma in DJ-1 KO mice
Abstract
Background: Growth hormone (GH) mainly serves an endocrine function to regulate somatic growth, but also serves an autocrine function in lung growth and pulmonary function. Several recent studies have demonstrated the role of autocrine GH in tumor progression in some organs. However, it is not clear whether excessive secretion of GH in the lungs is related to pulmonary nodule formation.
Methods: Firstly, the lung tissues dissected from mice were used for Western blotting and PCR measurement. Secondly, the cultured cells were used for examining effects of GH on B16F10 murine melanoma cells. Thirdly, male C57BL/6 mice were intravenously injected with B16F10 cells and then subcutaneously injected with recombinant GH twice per week for three weeks. Finally, stably transfected pool of B16F10 cells with knockdown of growth hormone receptor (GHR) was used to be injected into mice.
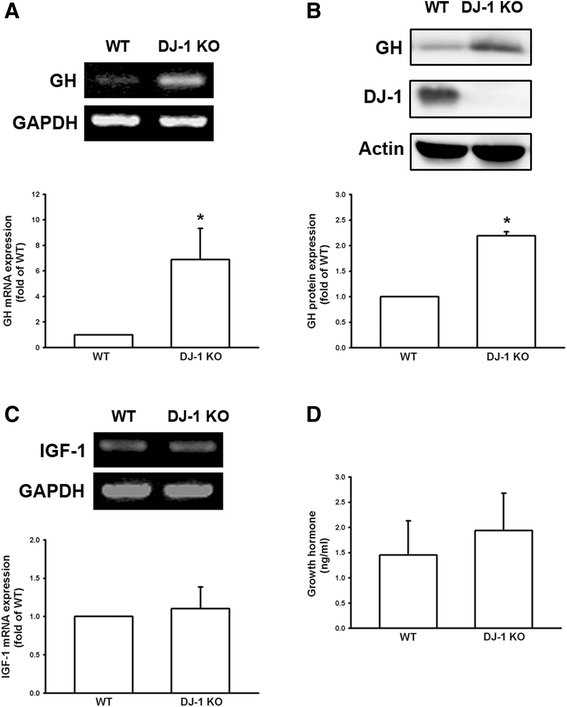
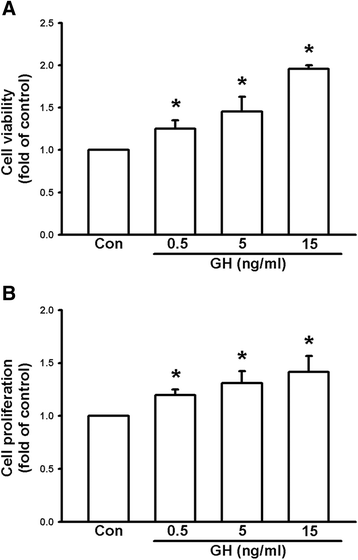
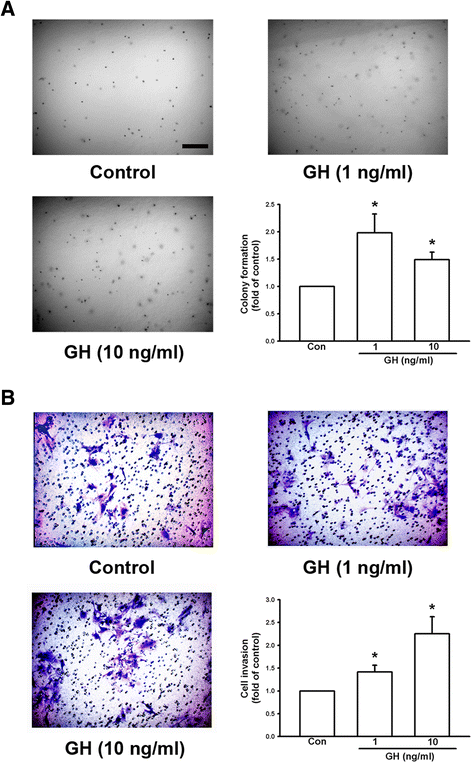
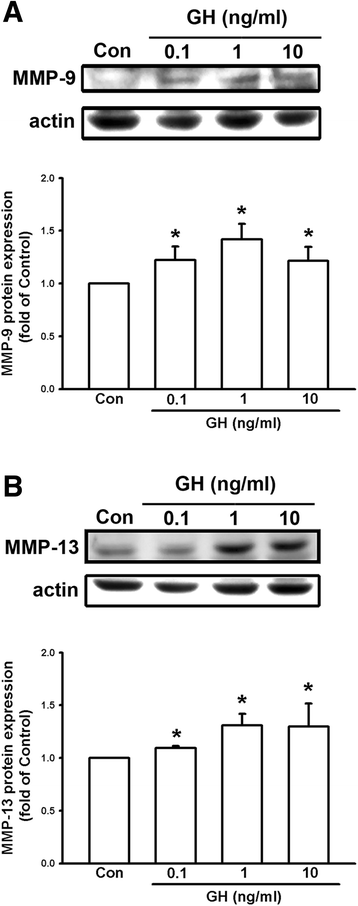
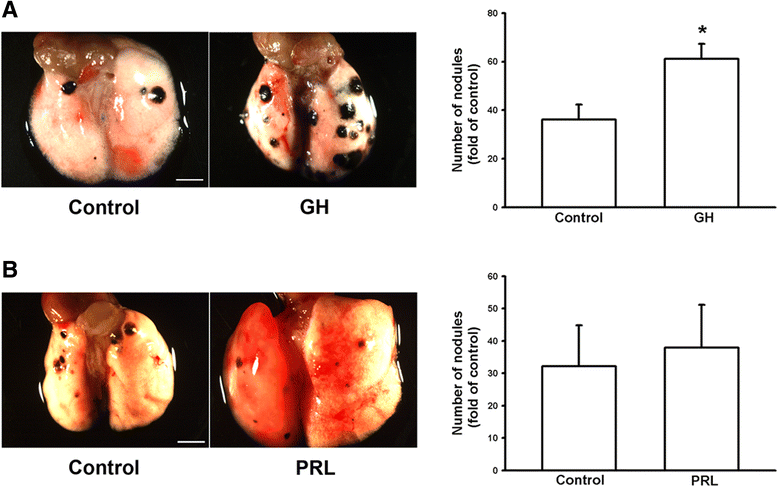
Results: We found that expression of GH was elevated in the lungs of DJ-1 knockout (KO) mice. We also examined the effects of GH on the growth of cultured melanoma cells. The results showed that GH increased proliferation, colony formation, and invasive capacity of B16F10 cells. In addition, GH also increased the expression of matrix metalloproteinases (MMPs) in B16F10 cells. Administration of GH in vivo enhanced lung nodule formation in C57/B6 mice. Increased lung nodule formation in DJ-1 KO mice following intravenous injection of melanoma cells was inhibited by GHR knockdown in B16F10 cells.
Conclusions: These results indicate that up-regulation of GH in the lungs of DJ-1 KO mice may enhance the malignancy of B16F10 cells and nodule formation in pulmonary metastasis of melanoma.
Keywords: Growth hormone; Knockout mice; Lung metastasis; Melanoma; Oncogenesis.
Figures


References
-
- Le Naour F, Misek DE, Krause MC, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7(11):3328–35. - PubMed
-
- Pardo M, Garcia A, Thomas B, et al. The characterization of the invasion phenotype of uveal melanoma tumour cells shows the presence of MUC18 and HMG-1 metastasis markers and leads to the identification of DJ-1 as a potential serum biomarker. Int J Cancer. 2006;119(5):1014–22. doi: 10.1002/ijc.21942. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

