The RUDY study platform - a novel approach to patient driven research in rare musculoskeletal diseases
- PMID: 27825362
- PMCID: PMC5101709
- DOI: 10.1186/s13023-016-0528-6
The RUDY study platform - a novel approach to patient driven research in rare musculoskeletal diseases
Abstract
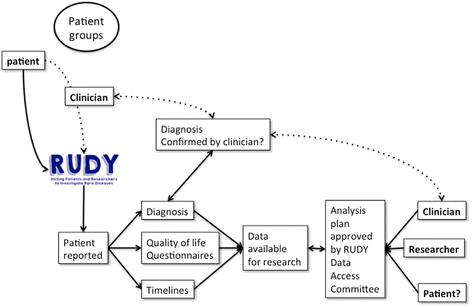
Background: Research into rare diseases is becoming more common, with recognition of the significant diagnostic and therapeutic care gaps. Registries are considered a key research methodology to address rare diseases. This report describes the structure of the Rare UK Diseases Study (RUDY) platform that aims to improve research processes and address many of the challenges of carrying out rare musculoskeletal disease research. RUDY is an internet-based platform with online registration, initial verbal consent, online capture of patient reported outcome measures and events within a dynamic consent framework. The database structure, security and governance framework are described.
Results: There have been 380 participants recruited into RUDY with completed questionnaire rates in excess of 50 %. There has been one withdrawal and two participants have amended their consent options.
Conclusions: The strengths of RUDY include low burden for the clinical team, low research administration costs with high participant recruitment and ease of data collection and access. This platform has the potential to be used as the model for other rare diseases globally.
Keywords: Database management systems; Dynamic consent; Patient reported outcome measures; Rare diseases.
Figures
References
-
- Cavero-Carbonell C, Gras-Colomer E, Guaita-Calatrava R, Lopez-Briones C, Amoros R, Abaitua I, et al. Consensus on the criteria needed for creating a rare-disease patient registry. A Delphi study. J Public Health (Oxf). 2015;38(2):e178–e186. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical