Siderophores in Iron Metabolism: From Mechanism to Therapy Potential
- PMID: 27825668
- PMCID: PMC5135587
- DOI: 10.1016/j.molmed.2016.10.005
Siderophores in Iron Metabolism: From Mechanism to Therapy Potential
Abstract
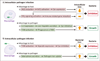
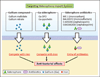
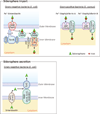
Iron is an essential nutrient for life. During infection, a fierce battle of iron acquisition occurs between the host and bacterial pathogens. Bacteria acquire iron by secreting siderophores, small ferric iron-binding molecules. In response, host immune cells secrete lipocalin 2 (also known as siderocalin), a siderophore-binding protein, to prevent bacterial reuptake of iron-loaded siderophores. To counter this threat, some bacteria can produce lipocalin 2-resistant siderophores. This review discusses the recently described molecular mechanisms of siderophore iron trafficking between host and bacteria, highlighting the therapeutic potential of exploiting pathogen siderophore machinery for the treatment of antibiotic-resistant bacterial infections. Because the latter reflect a persistent problem in hospital settings, siderophore-targeting or siderophore-based compounds represent a promising avenue to combat such infections.
Keywords: Lcn2; hypoxia; iron; mitophagy; siderocalin; siderophore.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

