Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years
- PMID: 27825687
- PMCID: PMC5568093
- DOI: 10.1016/j.athoracsur.2016.08.071
Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years
Abstract
Background: The purpose of this study was to assess survival for patients with malignant pleural mesothelioma (MPM), epithelial subtype, utilizing extended pleurectomy-decortication combined with intraoperative photodynamic therapy (PDT) and adjuvant pemetrexed-based chemotherapy.
Methods: From 2005 to 2013, 90 patients underwent lung-sparing surgery and PDT for MPM. All patients had a preoperative diagnosis of epithelial subtype, of which 17 proved to be of mixed histology. The remaining 73 patients with pure epithelial subtype were analyzed. All patients received lung-sparing surgery and PDT; 92% also received chemotherapy. The median follow-up was 5.3 years for living patients.
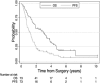
Results: Macroscopic complete resection was achieved in all 73 patients. Thirty-day mortality was 3% and 90-day mortality was 4%. For all 73 patients (89% American Joint Commission on Cancer stage III/IV, 69% N2 disease, median tumor volume 550 mL), the median overall and disease-free survivals were 3 years and 1.2 years, respectively. For the 19 patients without lymph node metastases (74% stage III/IV, median tumor volume 325 mL), the median overall and disease-free survivals were 7.3 years and 2.3 years, respectively.
Conclusions: This is a mature dataset for MPM that demonstrates the ability to safely execute a complex treatment plan that included a surgical technique that consistently permitted achieving a macroscopic complete resection while preserving the lung. The role for lung-sparing surgery is unclear but this series demonstrates that it is an option, even for advanced cases. The overall survival of 7.3 years for the node negative subset of patients, still of advanced stage, is encouraging. Of particular interest is the overall survival being approximately triple the disease-free survival, perhaps PDT related. The impact of PDT is unclear, but it is hoped that it will be established by an ongoing randomized trial.
Copyright © 2017 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Pleurectomy/decortication for malignant pleural mesothelioma.J Thorac Dis. 2017 Mar;9(3):460-461. doi: 10.21037/jtd.2017.03.33. J Thorac Dis. 2017. PMID: 28449444 Free PMC article. No abstract available.
-
Malignant pleural mesothelioma: key determinants in tailoring the right treatment for the right patient.J Thorac Dis. 2017 Mar;9(3):485-489. doi: 10.21037/jtd.2017.03.27. J Thorac Dis. 2017. PMID: 28449451 Free PMC article. No abstract available.
-
Reply.Ann Thorac Surg. 2017 Oct;104(4):1434-1435. doi: 10.1016/j.athoracsur.2017.02.025. Ann Thorac Surg. 2017. PMID: 28935311 No abstract available.
-
New Keywords in the Treatment of Malignant Pleural Mesothelioma.Ann Thorac Surg. 2017 Oct;104(4):1434. doi: 10.1016/j.athoracsur.2016.12.007. Ann Thorac Surg. 2017. PMID: 28935312 No abstract available.
References
-
- Damhuis RA, Khakwani A, DeSchutter H, Rich AI, Burgers JA, vanMeerbeeck JP. Treatment patterns and survival analysis in 9014 patients with malignant pleural mesothelioma from Belgium, the Netherlands and England. Lung Cancer. 2015;(89):212–217. - PubMed
-
- Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomized, controlled, open-label, phase 3 trial. Lancet. 2015 Dec 21;:1–10. - PubMed
-
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. - PubMed
-
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol. 2011;6:1304–1312. - PubMed
-
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2008;136:605. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

