The Gametocytocidal Efficacy of Different Single Doses of Primaquine with Dihydroartemisinin-piperaquine in Asymptomatic Parasite Carriers in The Gambia: A Randomized Controlled Trial
- PMID: 27825738
- PMCID: PMC5264436
- DOI: 10.1016/j.ebiom.2016.10.032
The Gametocytocidal Efficacy of Different Single Doses of Primaquine with Dihydroartemisinin-piperaquine in Asymptomatic Parasite Carriers in The Gambia: A Randomized Controlled Trial
Abstract
Background: Asymptomatic low-density gametocyte carriers represent the majority of malaria-infected individuals. However, the impact of recommended treatment with single low dose of primaquine and an artemisinin-based combination therapy to reduce transmission in this group is unknown.
Methods: This was a four-arm, open label, randomized controlled trial comparing the effect of dihydroartemisinin-piperaquine (DHAP) alone or combined with single dose of primaquine (PQ) at 0.20mg/kg, 0.40mg/kg, or 0.75mg/kg on Plasmodium falciparum gametocytaemia, infectiousness to mosquitoes and hemoglobin change in asymptomatic, malaria-infected, glucose-6-phosphate dehydrogenase (G6PD) normal individuals. Randomization was done using a computer-generated sequence of uneven block sizes with codes concealed in sequentially numbered opaque envelopes. The primary endpoint was the prevalence of P. falciparum gametocytemia at day 7 of follow-up determined by quantitative nucleic acid sequence based assay and analysis was by intention to treat. The trial has been concluded (registration number: NCT01838902; https://clinicaltrials.gov/ct2/show/NCT01838902).
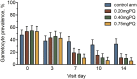
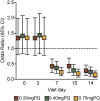
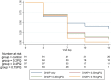
Results: A total of 694 asymptomatic, malaria-infected individuals were enrolled. Gametocyte prevalence at day 7 was 37.0% (54/146; 95% CI 29.2-45.4), 19.0% (27/142; 95% CI 12.9-26.4), 17.2% (25/145; 95% CI 11.0-23.5) and 10.6% (15/141; 95% CI 6.1-16.9) in the DHAP alone, 0.20mg/kg, 0.40mg/kg, and 0.75mg/kg PQ arms, respectively. The main adverse events reported include headache (130/471, 27.6%), cough (73/471, 15.5%), history of fever (61/471, 13.0%) and abdominal pain (57/471, 12.1%). There were five serious adverse events however, none was related to the interventions.
Interpretation: A single course of PQ significantly reduces gametocyte carriage in malaria-infected asymptomatic, G6PD-normal individuals without increasing the risk of clinical anemia. The limited number of successful mosquito infections suggests that post-treatment transmission potential in this asymptomatic population is low.
Keywords: Asymptomatic infection; Efficacy; Gametocyte carriage; Infectivity; Malaria; Plasmodium falciparum; Primaquine; Randomized trial.
Copyright © 2016 The Author. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Alves F., Gil L., Marrelli M., Ribolla P., Camargo E., Da silva L. Asymptomatic carriers of Plasmodium sp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J. Med. Entomol. 2005;42:777–779. - PubMed
-
- Bennett J.W., Pybus B.S., Yadava A., Tosh D., Sousa J.C., Mccarthy W.F., Deye G., Melendez V., Ockenhouse C.F. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N. Engl. J. Med. 2013;369:1381–1382. - PubMed
-
- Bereczky S., Montgomery S.M., Troye-blomberg M., Rooth I., Shaw M.A., Farnert A. Elevated anti-malarial IgE in asymptomatic individuals is associated with reduced risk for subsequent clinical malaria. Int. J. Parasitol. 2004;34:935–942. - PubMed
-
- Bousema T., Dinglasan R.R., Morlais I., Gouagna L.C., Van Warmerdam T., Awono-Ambene P.H., Bonnet S., Diallo M., Coulibaly M., Tchuinkam T., Mulder B., Targett G., Drakeley C., Sutherland C., Robert V., Doumbo O., Touré Y., Graves P.M., Roeffen W., Sauerwein R., Birkett A., Locke E., Morin M., Wu Y., Churcher T.S. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One. 2012;7 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

