Synthesis, anti-inflammatory, analgesic, COX1/2-inhibitory activity, and molecular docking studies of hybrid pyrazole analogues
- PMID: 27826185
- PMCID: PMC5096746
- DOI: 10.2147/DDDT.S118297
Synthesis, anti-inflammatory, analgesic, COX1/2-inhibitory activity, and molecular docking studies of hybrid pyrazole analogues
Abstract
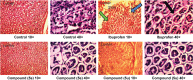
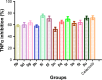
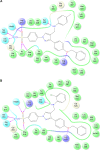
This article reports on the design, synthesis, and pharmacological activity of a new series of hybrid pyrazole analogues: 5a-5u. Among the series 5a-5u, the compounds 5u and 5s exhibited potent anti-inflammatory activity of 80.63% and 78.09% and inhibition of 80.87% and 76.56% compared with the standard drug ibuprofen, which showed 81.32% and 79.23% inhibition after 3 and 4 hours, respectively. On the basis of in vivo studies, 12 compounds were selected for assessment of their in vitro inhibitory action against COX1/2 and TNFα. The compounds 5u and 5s showed high COX2-inhibitory activity, with half-maximal inhibitory concentrations of 1.79 and 2.51 μM and selectivity index values of 72.73 and 65.75, respectively, comparable to celecoxib (selectivity index =78.06). These selected compounds were also tested for TNFα, cytotoxicity, and ulcerogenicity. Docking studies were also carried out to determine possible interactions of the potent compounds (5u and 5s), which also showed high docking scores of -12.907 and -12.24 compared to celecoxib, with a -9.924 docking score. These selective COX2 inhibitors were docked into the active site of COX2, and showed the same orientation and binding mode to that of celecoxib (selective COX2 inhibitor). Docking studies also showed that the SO2NH2 of 5u and 5s is inserted deep inside the selective pocket of the COX2-active site and formed a hydrogen-bond interaction with His90, Arg513, Phe518, Ser353, Gln192, and Ile517, which was further validated by superimposed docked pose with celecoxib.
Keywords: TNFα inhibition; analgesic activity; anti-inflammatory activity; molecular docking studies; pyrazole; selective COX2 inhibition.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
-
- Botting JH. Nonsteroidal antiinflammatory agents. Drugs Today (Barc) 1999;35:225–235. - PubMed
-
- Girgis AS, Ellithey M. Facile synthesis of non-steroidal anti-inflammatory active bisbenzamide-containing compounds. Bioorg Med Chem. 2006;14:8527–8532. - PubMed
-
- Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. - PubMed
-
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

