Analog Memristive Synapse in Spiking Networks Implementing Unsupervised Learning
- PMID: 27826226
- PMCID: PMC5078263
- DOI: 10.3389/fnins.2016.00482
Analog Memristive Synapse in Spiking Networks Implementing Unsupervised Learning
Abstract
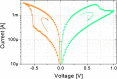
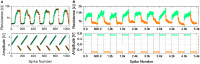
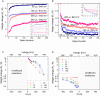
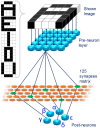
Emerging brain-inspired architectures call for devices that can emulate the functionality of biological synapses in order to implement new efficient computational schemes able to solve ill-posed problems. Various devices and solutions are still under investigation and, in this respect, a challenge is opened to the researchers in the field. Indeed, the optimal candidate is a device able to reproduce the complete functionality of a synapse, i.e., the typical synaptic process underlying learning in biological systems (activity-dependent synaptic plasticity). This implies a device able to change its resistance (synaptic strength, or weight) upon proper electrical stimuli (synaptic activity) and showing several stable resistive states throughout its dynamic range (analog behavior). Moreover, it should be able to perform spike timing dependent plasticity (STDP), an associative homosynaptic plasticity learning rule based on the delay time between the two firing neurons the synapse is connected to. This rule is a fundamental learning protocol in state-of-art networks, because it allows unsupervised learning. Notwithstanding this fact, STDP-based unsupervised learning has been proposed several times mainly for binary synapses rather than multilevel synapses composed of many binary memristors. This paper proposes an HfO2-based analog memristor as a synaptic element which performs STDP within a small spiking neuromorphic network operating unsupervised learning for character recognition. The trained network is able to recognize five characters even in case incomplete or noisy images are displayed and it is robust to a device-to-device variability of up to ±30%.
Keywords: HfO2; artificial synapse; memristor; resistive switching; spike time dependent plasticity; spiking neuromorphic network; synaptic plasticity; unsupervised learning.
Figures



References
-
- Ambrogio S., Balatti S., Milo V., Carboni R., Wang Z. Q., Calderoni A., et al. (2016a). Neuromorphic learning and recognition with one-transistor-one-resistor synapses and bistable metal oxide RRAM. IEEE Trans. Electr. Dev. 63, 1508–1515. 10.1109/TED.2016.2526647 - DOI
-
- Berdan R., Serb A., Khiat A., Regoutz A., Papavassiliou C., Prodromakis T. (2015). A μ-controller-based system for interfacing selector-less RRAM crossbar arrays. IEEE Trans. Electr. Dev. 62, 2190–2196. 10.1109/TED.2015.2433676 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

