Potentiation of Schaffer-Collateral CA1 Synaptic Transmission by eEF2K and p38 MAPK Mediated Mechanisms
- PMID: 27826228
- PMCID: PMC5078695
- DOI: 10.3389/fncel.2016.00247
Potentiation of Schaffer-Collateral CA1 Synaptic Transmission by eEF2K and p38 MAPK Mediated Mechanisms
Abstract
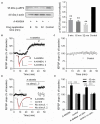
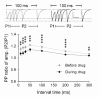
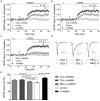
The elongation factor 2 kinase (eEF2K), likewise known as CaMKIII, has been demonstrated to be involved in antidepressant responses of NMDA receptor antagonists. Even so, it remains open whether direct inhibition of eEF2K without altering up-stream or other signaling pathways affects hippocampal synaptic transmission and neuronal network synchrony. Inhibition of eEF2K by the selective and potent eEF2K inhibitor A-484954 induced a fast pre-synaptically mediated enhancement of synaptic transmission and synchronization of neural network activity. The eEF2K-inhibition mediated potentiation of synaptic transmission of hippocampal CA1 neurons is most notably independent of protein synthesis and does not rely on protein kinase C, protein kinase A or mitogen-activated protein kinase (MAPK)/extracellular signal-regulated protein kinase 1/2. Moreover, the strengthening of synaptic transmission in the response to the inhibition of eEF2K was strongly attenuated by the inhibition of p38 MAPK. In addition, we show the involvement of barium-sensitive and more specific the TWIK-related potassium-1 (TREK-1) channels in the eEF2K-inhibition mediated potentiation of synaptic transmission. These findings reveal a novel pathway of eEF2K mediated regulation of hippocampal synaptic transmission. Further research is required to study whether such compounds could be beneficial for the development of mood disorder treatments with a fast-acting antidepressant response.
Keywords: MAPK; eEF2; hippocampus; memory; oscillation; protein synthesis; synaptic plasticity.
Figures









References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

