The Pharmacological Potential of Non-ribosomal Peptides from Marine Sponge and Tunicates
- PMID: 27826240
- PMCID: PMC5078478
- DOI: 10.3389/fphar.2016.00333
The Pharmacological Potential of Non-ribosomal Peptides from Marine Sponge and Tunicates
Abstract
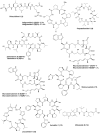
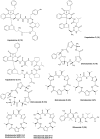
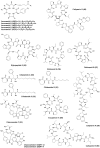
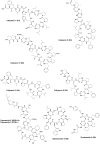
Marine biodiversity is recognized by a wide and unique array of fascinating structures. The complex associations of marine microorganisms, especially with sponges, bryozoans, and tunicates, make it extremely difficult to define the biosynthetic source of marine natural products or to deduce their ecological significance. Marine sponges and tunicates are important source of novel compounds for drug discovery and development. Majority of these compounds are nitrogen containing and belong to non-ribosomal peptide (NRPs) or mixed polyketide-NRP natural products. Several of these peptides are currently under trial for developing new drugs against various disease areas, including inflammatory, cancer, neurodegenerative disorders, and infectious disease. This review features pharmacologically active NRPs from marine sponge and tunicates based on their biological activities.
Keywords: marine ecosystem; marine natural products; non-ribosomal peptides; pharmacology; sponge; tunicates.
Figures


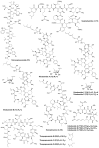
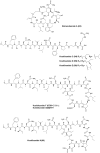


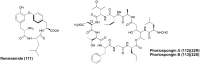
References
-
- Aoki S., Cao L., Matsui K., Rachmat R., Akiyama S.-I., Kobayashi M. (2004). Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron 60, 7053–7059. 10.1016/j.tet.2003.07.020 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

