Fluoxetine Prevents Aβ1-42-Induced Toxicity via a Paracrine Signaling Mediated by Transforming-Growth-Factor-β1
- PMID: 27826242
- PMCID: PMC5078904
- DOI: 10.3389/fphar.2016.00389
Fluoxetine Prevents Aβ1-42-Induced Toxicity via a Paracrine Signaling Mediated by Transforming-Growth-Factor-β1
Abstract
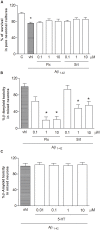
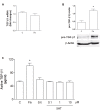
Selective reuptake inhibitors (SSRIs), such as fluoxetine and sertraline, increase circulating Transforming-Growth-Factor-β1 (TGF-β1) levels in depressed patients, and are currently studied for their neuroprotective properties in Alzheimer's disease. TGF-β1 is an anti-inflammatory cytokine that exerts neuroprotective effects against β-amyloid (Aβ)-induced neurodegeneration. In the present work, the SSRI, fluoxetine, was tested for the ability to protect cortical neurons against 1 μM oligomeric Aβ1-42-induced toxicity. At therapeutic concentrations (100 nM-1 μM), fluoxetine significantly prevented Aβ-induced toxicity in mixed glia-neuronal cultures, but not in pure neuronal cultures. Though to a lesser extent, also sertraline was neuroprotective in mixed cultures, whereas serotonin (10 nM-10 μM) did not mimick fluoxetine effects. Glia-conditioned medium collected from astrocytes challenged with fluoxetine protected pure cortical neurons against Aβ toxicity. The effect was lost in the presence of a neutralizing antibody against TGF-β1 in the conditioned medium, or when the specific inhibitor of type-1 TGF-β1 receptor, SB431542, was added to pure neuronal cultures. Accordingly, a 24 h treatment of cortical astrocytes with fluoxetine promoted the release of active TGF-β1 in the culture media through the conversion of latent TGF-β1 to mature TGF-β1. Unlike fluoxetine, both serotonin and sertraline did not stimulate the astrocyte release of active TGF-β1. We conclude that fluoxetine is neuroprotective against Aβ toxicity via a paracrine signaling mediated by TGF-β1, which does not result from a simplistic SERT blockade.
Keywords: Alzheimer’s disease; TGF-β1; antidepressants; cortical neurons; depression; fluoxetine; neuroprotection; β-amyloid.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

