Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs
- PMID: 27826359
- PMCID: PMC5100090
- DOI: 10.1186/s13099-016-0136-y
Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs
Abstract
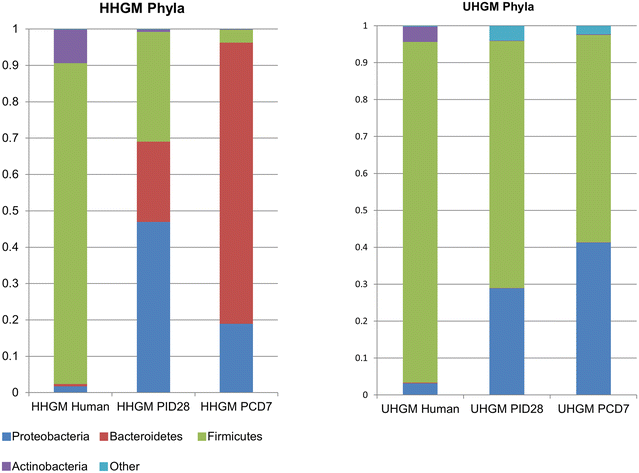
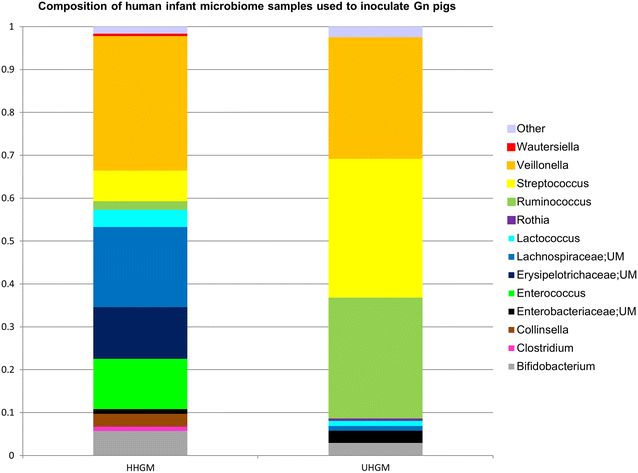
Background: Rotavirus vaccines have poor efficacy in infants from low- and middle-income countries. Gut microbiota is thought to influence the immune response to oral vaccines. Thus, we developed a gnotobiotic (Gn) pig model of enteric dysbiosis to study the effects of human gut microbiota (HGM) on immune responses to rotavirus vaccination, and the effects of rotavirus challenge on the HGM by colonizing Gn pigs with healthy HGM (HHGM) or unhealthy HGM (UHGM). The UHGM was from a Nicaraguan infant with a high enteropathy score (ES) and no seroconversion following administration of oral rotavirus vaccine, while the converse was characteristic of the HHGM. Pigs were vaccinated, a subset was challenged, and immune responses and gut microbiota were evaluated.
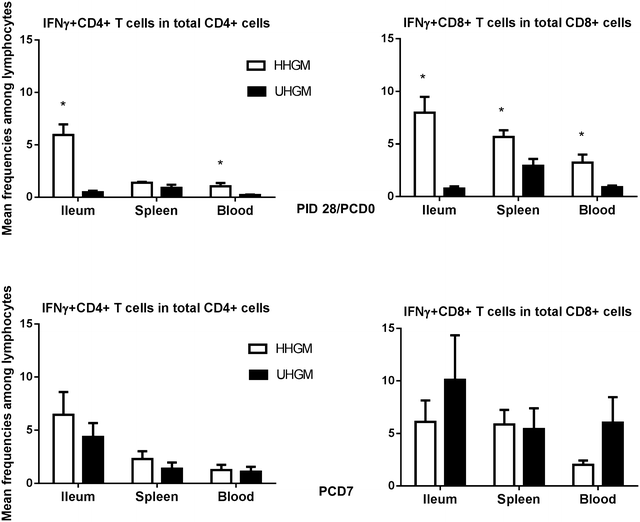
Results: Significantly more rotavirus-specific IFN-γ producing T cells were in the ileum, spleen, and blood of HHGM than those in UHGM pigs after three vaccine doses, suggesting HHGM induces stronger cell-mediated immunity than UHGM. There were significant correlations between multiple Operational Taxonomic Units (OTUs) and frequencies of IFN-γ producing T cells at the time of challenge. There were significant positive correlations between Collinsella and CD8+ T cells in blood and ileum, as well as CD4+ T cells in blood, whereas significant negative correlations between Clostridium and Anaerococcus, and ileal CD8+ and CD4+ T cells. Differences in alpha diversity and relative abundances of OTUs were detected between the groups both before and after rotavirus challenge.
Conclusion: Alterations in microbiome diversity and composition along with correlations between certain microbial taxa and T cell responses warrant further investigation into the role of the gut microbiota and certain microbial species on enteric immunity. Our results support the use of HGM transplanted Gn pigs as a model of human dysbiosis during enteric infection, and oral vaccine responses.
Keywords: Enteric dysbiosis; Enteric immunity; Gnotobiotic pig; Rotavirus; Vaccine.
Figures





References
-
- Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec. 2013, 88(5):49–64. - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. Network WH-cGRS: 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–141. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–614. doi: 10.1016/S0140-6736(10)60889-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

