Cysteine-independent activation/inhibition of heme oxygenase-2
- PMID: 27826418
- PMCID: PMC5075677
- DOI: 10.4103/2045-9912.179341
Cysteine-independent activation/inhibition of heme oxygenase-2
Abstract
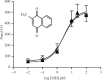
Reactive thiols of cysteine (cys) residues in proteins play a key role in transforming chemical reactivity into a biological response. The heme oxygenase-2 (HO-2) isozyme contains two cys residues that have been implicated in binding of heme and also the regulation of its activity. In this paper, we address the question of a role for cys residues for the HO-2 inhibitors or activators designed in our laboratory. We tested the activity of full length recombinant human heme oxygenase-2 (FL-hHO-2) and its analog in which cys265 and cys282 were both replaced by alanine to determine the effect on activation by menadione (MD) and inhibition by QC-2350. Similar inhibition by QC-2350 and almost identical activation by MD was observed for both recombinant FL-hHO-2s. Our findings are interpreted to mean that thiols of FL-hHO-2s are not involved in HO-2 activation or inhibition by the compounds that have been designed and identified by us. Activation or inhibition of HO-2 by our compounds should be attributed to a mechanism other than altering binding affinity of HO-2 for heme through cys265 and cys282.
Keywords: HO-2 activator; HO-2 inhibitor; Heme degradation; QC-2350; heme oxygenase-2; in vitro; menadione; thiols.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, Tada M, Fujii S, Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide. Implication for cytotoxicity. J Biol Chem. 1995;270:21035–21039. - PubMed
-
- Ikeda Y, Noguchi T. Allosteric regulation of pyruvate kinase M2 isozyme involves a cysteine residue in the intersubunit contact. J Biol Chem. 1998;273:12227–12233. - PubMed
-
- Kong X, Vukomanovic D, Nakatsu K, Szarek WA. Structureactivity relationships of 1,2-disubstituted benzimidazoles: selective inhibition of heme oxygenase-2 activity. ChemMedChem. 10:1435–1441. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

