Preparation of alginate-chitosan-cyclodextrin micro- and nanoparticles loaded with anti-tuberculosis compounds
- PMID: 27826495
- PMCID: PMC5082317
- DOI: 10.3762/bjnano.7.112
Preparation of alginate-chitosan-cyclodextrin micro- and nanoparticles loaded with anti-tuberculosis compounds
Abstract
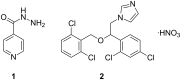
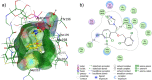
This paper describes the synthesis and application of alginate-chitosan-cyclodextrin micro- and nanoparticulate systems loaded with isoniazid (INH) and isoconazole nitrate (ISN) as antimycobacterial compounds. Preparation and morphology of the obtained particles, as well as antimycobacterial activity data of the obtained systems are presented. Docking of isoconazole into the active site of enoyl-acyl carrier protein reductase (InhA) of Mycobacetrium tuberculosis was carried out in order to predict the binding affinity and non-covalent interactions stabilizing the InhA-isoconazole complex. To assess these interactions, frontier molecular orbital calculations were performed for the active site of InhA and isoconazole obtained from docking. Isoconazole was predicted to be an active inhibitor of InhA with the analysis of the molecular docking and electron density distribution. It has been detected that alginate-chitosan-cyclodextrin microparticulate systems loaded with INH and ISN are as effective as pure INH applied in higher dosages.
Keywords: chitosan; density functional theory (DFT); isoconazole; isoniazid; molecular docking; quaternary system; sodium alginate; β-cyclodextrin.
Figures








References
-
- [May 31;2016 ];Global Tuberculosis Report 2015, 20th edition, World Health Organization, Geneva, Switzerland. Available from: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.
-
- Boldescu V, Macaev F, Duca G. Chem J Mold. 2014;9:8–13.
LinkOut - more resources
Full Text Sources
Other Literature Sources
