Living donor liver transplantation for hepatocellular carcinoma at the University of Tokyo Hospital
- PMID: 27826554
- PMCID: PMC5075828
- DOI: 10.21037/hbsn.2016.08.05
Living donor liver transplantation for hepatocellular carcinoma at the University of Tokyo Hospital
Erratum in
-
Erratum to Living donor liver transplantation for hepatocellular carcinoma at the University of Tokyo Hospital.Hepatobiliary Surg Nutr. 2016 Dec;5(6):523. doi: 10.21037/hbsn.2016.11.03. Hepatobiliary Surg Nutr. 2016. PMID: 28124014 Free PMC article.
Abstract
Background: Living donor liver transplantation (LDLT) is an established treatment not only for those with end-stage liver disease but for those with hepatocellular carcinoma (HCC) developing in cirrhotic liver. The aim of this study was to present a single-center experience of LDLT for HCC at the University of Tokyo Hospital, Japan.
Methods: Among 573 liver transplant recipients from January 1996 until the end of 2015, 139 patients have been indicated LDLT for the treatment of HCC, and were the subjects of the present study. We use the expanded criteria for HCC as follows; the number of tumor should be five or less, and the maximum diameter of the tumor should be 5 cm or less, without the distant metastasis nor the vascular invasion (Tokyo criteria, 5-5 rule). We also focused on the identification of the incidental intrahepatic cholangiocarcinoma (ICC) and combined hepatocellular carcinoma/cholangiocarcinoma (cHCC-CC) in liver explants.
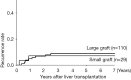
Results: The overall 1-, 5-, and 10-year recurrence-free and patient survival rates were 95%, 91%, and 91%, 91%, and 80%, 78%, respectively. The 1-, 3-, and 5-year cumulative recurrence rate was 5%, 6%, and 6% for within Milan, 0%, 8%, and 8% for beyond Milan/within Tokyo, and 33%, 50%, and 50% for beyond Tokyo, respectively, demonstrating the significantly impaired outcome of those beyond Tokyo criteria (P<0.001). The high alpha-fetoprotein (AFP) value (≥400 ng/mL), the high des-gamma-carboxy prothrombin (DCP) value (≥200 mAU/mL) and beyond the Tokyo criteria were proved to be significant predictors for the HCC recurrence, but the size or the type of the partial graft was not associated. Incidental ICC and cHCC-CC were found in one and two patients, respectively, with the size of less than 2 cm in all cases. ICC was not detected in preoperative evaluation but cHCC-CCs were misdiagnosed as HCC preoperatively. All three patients were alive without recurrence with a follow-up period of 2 to 14 years.
Conclusions: The present results of our institution seem acceptable in terms of the recurrence-free and patient survival. The issues of the expansion of indication, living donor vs. deceased donor for HCC, and liver transplantation (LT) for cholangiocarcinoma are still left to be investigated in future studies.
Keywords: Liver transplantation (LT); combined hepatocellular carcinoma/cholangiocarcinoma (cHCC-CC); hepatocellular carcinoma (HCC); incidental intrahepatic cholangiocarcinoma (ICC); living donor liver transplantation (LDLT).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
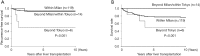
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
