CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2
- PMID: 27827367
- PMCID: PMC5105190
- DOI: 10.1038/ncomms13346
CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2
Abstract
Tissue-resident memory CD8+ T (CD8 TRM) cells are an essential component of protective immune responses at barrier tissues, including the female genital tract. However, the mechanisms that lead to the initiation of CD8 TRM-mediated protective immunity after viral infection are unclear. Here we report that CD8 TRM cells established by 'prime and pull' method confer protection against genital HSV-2 infection, and that IFN-γ produced by CD8 TRM cells is required for this protection. Furthermore, we find that CD8 TRM-cell restimulation depends on a population of CD301b+ antigen-presenting cells (APC) in the lamina propria. Elimination of MHC class I on CD301b+ dendritic cells abrogates protective immunity, suggesting the requirement for cognate antigen presentation to CD8 TRM cells by CD301b+ dendritic cells. These results define the requirements for CD8 TRM cells in protection against genital HSV-2 infection and identify the population of APC that are responsible for activating these cells.
Figures



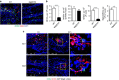

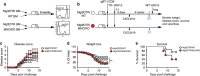
References
-
- Gebhardt T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009). - PubMed
-
- Mackay L. K. et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

