Ethanol-Sensitive Pacemaker Neurons in the Mouse External Globus Pallidus
- PMID: 27827370
- PMCID: PMC5506786
- DOI: 10.1038/npp.2016.251
Ethanol-Sensitive Pacemaker Neurons in the Mouse External Globus Pallidus
Abstract
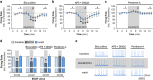
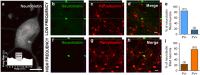
Although ethanol is one of the most widely used drugs, we still lack a full understanding of which neuronal subtypes are affected by this drug. Pacemaker neurons exert powerful control over brain circuit function, but little is known about ethanol effects on these types of neurons. Neurons in the external globus pallidus (GPe) generate pacemaker activity that controls basal ganglia, circuitry associated with habitual and compulsive drug use. We performed patch-clamp recordings from GPe neurons and found that bath application of ethanol dose-dependently decreased the firing rate of low-frequency GPe neurons, but did not alter the firing of high-frequency neurons. GABA or glutamate receptor antagonists did not block the ethanol effect. The GPe is comprised of a heterogeneous population of neurons. We used Lhx6-EGFP and Npas1-tdTm mice strains to identify low-frequency neurons. Lhx6 and Npas1 neurons exhibited decreased firing with ethanol, but only Npas1 neurons were sensitive to 10 mM ethanol. Large-conductance voltage and Ca2+-activated K+ (BK) channel have a key role in the ethanol effect on GPe neurons, as the application of BK channel inhibitors blocked the ethanol-induced firing decrease. Ethanol also increased BK channel open probability measured in single-channel recordings from Npas1-tdTm neurons. In addition, in vivo electrophysiological recordings from GPe showed that ethanol decreased the firing of a large subset of low-frequency neurons. These findings indicate how selectivity of ethanol effects on pacemaker neurons can occur, and enhance our understanding of the mechanisms contributing to acute ethanol effects on the basal ganglia.
Figures




References
-
- Albin RL, Young AB, Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

