Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration
- PMID: 27827394
- PMCID: PMC5101530
- DOI: 10.1038/srep36570
Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration
Abstract
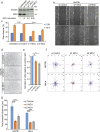
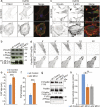
Intracellular Ca2+ signaling regulates cell migration by acting on cytoskeleton architecture, cell directionality and focal adhesions dynamics. In migrating cells, cytosolic Ca2+ pool and Ca2+ pulses are described as key components of these effects. Whereas the role of the mitochondrial calcium homeostasis and the Mitochondria Cacium Uniporter (MCU) in cell migration were recently highlighted in vivo using the zebrafish model, their implication in actin cystokeleton dynamics and cell migration in mammals is not totally characterized. Here, we show that mcu silencing in two human cell lines compromises their migration capacities. This phenotype is characterized by actin cytoskeleton stiffness, a cell polarization loss and an impairment of the focal adhesion proteins dynamics. At the molecular level, these effects appear to be mediated by the reduction of the ER and cytosolic Ca2+ pools, which leads to a decrease in Rho-GTPases, RhoA and Rac1, and Ca2+-dependent Calpain activites, but seem to be independent of intracellular ATP levels. Together, this study highlights the fundamental and evolutionary conserved role of the mitochondrial Ca2+ homeostasis in cytoskeleton dynamics and cell migration.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

