Lactate is a potent inhibitor of the capsaicin receptor TRPV1
- PMID: 27827430
- PMCID: PMC5101504
- DOI: 10.1038/srep36740
Lactate is a potent inhibitor of the capsaicin receptor TRPV1
Abstract
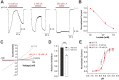
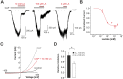
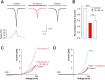
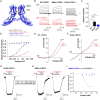
Tissue ischemia results in an accumulation of lactate and local or systemic lactic acidosis. In nociceptive sensory neurons, lactate was reported to sensitize or activate the transient receptor potential ion channel TRPA1 and acid-sensing ion channels (ASICs). However, it is unclear how lactate modulates the TRPV1 regarded as the main sensor for acidosis in sensory neurons. In this study we investigated the effects of lactate (LA) on recombinant and native TRPV1 channels and on TRPV1-mediated release of neuropeptides from mouse nerves. TRPV1-mediated membrane currents evoked by protons, capsaicin or heat are inhibited by LA at concentrations ranging from 3 μM to 100 mM. LA inhibits TRPV1-mediated proton-induced Ca2+-influx in dorsal root ganglion neurons as well as proton-evoked neuropeptide release from mouse nerves. Inhibition of TRPV1 by LA is significantly stronger on inward currents as compared to outward currents since LA affects channel gating, shifting the activation curve towards more positive potentials. The mutation I680A in the pore lower gate displays no LA inhibition. Cell-attached as well as excised inside- and outside-out patches suggest an interaction through an extracellular binding site. In conclusion, our data demonstrate that lactate at physiologically relevant concentrations is a potent endogenous inhibitor of TRPV1.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

