Natural Products from Microalgae with Potential against Alzheimer's Disease: Sulfolipids Are Potent Glutaminyl Cyclase Inhibitors
- PMID: 27827845
- PMCID: PMC5128746
- DOI: 10.3390/md14110203
Natural Products from Microalgae with Potential against Alzheimer's Disease: Sulfolipids Are Potent Glutaminyl Cyclase Inhibitors
Abstract
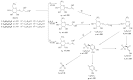
In recent years, many new enzymes, like glutaminyl cyclase (QC), could be associated with pathophysiological processes and represent targets for many diseases, so that enzyme-inhibiting properties of natural substances are becoming increasingly important. In different studies, the pathophysiology connection of QC to various diseases including Alzheimer's disease (AD) was described. Algae are known for the ability to synthesize complex and highly-diverse compounds with specific enzyme inhibition properties. Therefore, we screened different algae species for the presence of QC inhibiting metabolites using a new "Reverse Metabolomics" technique including an Activity-correlation Analysis (AcorA), which is based on the correlation of bioactivities to mass spectral data with the aid of mathematic informatics deconvolution. Thus, three QC inhibiting compounds from microalgae belonging to the family of sulfolipids were identified. The compounds showed a QC inhibition of 81% and 76% at concentrations of 0.25 mg/mL and 0.025 mg/mL, respectively. Thus, for the first time, sulfolipids are identified as QC inhibiting compounds and possess substructures with the required pharmacophore qualities. They represent a new lead structure for QC inhibitors.
Keywords: AD; Scenedesmus sp.; glutaminyl cyclase (QC) inhibitor; microalgae; natural product; reverse metabolomics; sulfolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Busby W.H., Quackenbush G.E., Humm J., Youngblood W.W., Kizers J.S. An Enzyme (s) That Converts Glutaminyl-peptides into pyroglutamyl-petides. J. Biol. Chem. 1987;262:8532–8536. - PubMed
-
- Schilling S., Niestroj A.J., Rahfeld J.-U., Hoffmann T., Wermann M., Zunkel K., Wasternack C., Demuth H.-U. Identification of human glutaminyl cyclase as a metalloenzyme. Potent inhibition by imidazole derivatives and heterocyclic chelators. J. Biol. Chem. 2003;278:49773–49779. doi: 10.1074/jbc.M309077200. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

