Efficacy and Safety of Vernakalant for Cardioversion of Recent-onset Atrial Fibrillation in the Asia-Pacific Region: A Phase 3 Randomized Controlled Trial
- PMID: 27828791
- PMCID: PMC5295492
- DOI: 10.1097/FJC.0000000000000445
Efficacy and Safety of Vernakalant for Cardioversion of Recent-onset Atrial Fibrillation in the Asia-Pacific Region: A Phase 3 Randomized Controlled Trial
Abstract
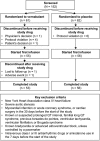
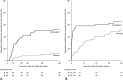
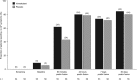
Atrial fibrillation (AF) is a common clinically significant cardiac arrhythmia. This phase 3 randomized, double-blind, placebo-controlled trial assessed the efficacy and safety of vernakalant hydrochloride for the pharmacological conversion of AF to sinus rhythm in patients with recent-onset (>3 hours to ≤7 days) symptomatic AF from the Asia-Pacific region. Patients received an infusion of vernakalant (3 mg/kg) or placebo for 10 minutes. If AF had not been terminated 15 minutes later, a second infusion of vernakalant (2 mg/kg) or placebo for 15 minutes was administered. The primary efficacy end point was conversion of AF to sinus rhythm for >1 minute within 90 minutes. The study was terminated early for administrative reasons; 123 patients from Korea, Taiwan, and India were randomized to receive vernakalant (n = 55) or placebo (n = 56). A greater proportion of patients who received vernakalant (52.7%) than placebo (12.5%) met the primary end point (P < 0.001), and cardioversion was faster in the vernakalant group than in the placebo group (P < 0.001). Vernakalant was generally well tolerated; the incidence of treatment-emergent adverse events was similar between the groups. We conclude that vernakalant is efficacious in the rapid cardioversion of recent-onset AF in patients from the Asia-Pacific region.
Figures
References
-
- Kodani E, Atarashi H. Prevalence of atrial fibrillation in Asia and the world. J Arrhythmia. 2012;28:330–337.
-
- Yap KB, Ng TP, Ong HY. Low prevalence of atrial fibrillation in community-dwelling Chinese aged 55 years or older in Singapore: a population-based study. J Electrocardiol. 2008;41:94–98. - PubMed
-
- Wen-Hang QI. Retrospective investigation of hospitalised patients with atrial fibrillation in mainland China. Int J Cardiol. 2005;105:283–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

