Antiretroviral Drug Use in a Cross-Sectional Population Survey in Africa: NIMH Project Accept (HPTN 043)
- PMID: 27828875
- PMCID: PMC5233465
- DOI: 10.1097/QAI.0000000000001229
Antiretroviral Drug Use in a Cross-Sectional Population Survey in Africa: NIMH Project Accept (HPTN 043)
Erratum in
-
Antiretroviral Drug Use in a Cross-Sectional Population Survey in Africa: NIMH Project Accept (HPTN 043): Erratum.J Acquir Immune Defic Syndr. 2018 Apr 15;77(5):e48. doi: 10.1097/QAI.0000000000001672. J Acquir Immune Defic Syndr. 2018. PMID: 29521876 No abstract available.
Abstract
Background: Antiretroviral (ARV) drug treatment benefits the treated individual and can prevent HIV transmission. We assessed ARV drug use in a community-randomized trial that evaluated the impact of behavioral interventions on HIV incidence.
Methods: Samples were collected in a cross-sectional survey after a 3-year intervention period. ARV drug testing was performed using samples from HIV-infected adults at 4 study sites (Zimbabwe; Tanzania; KwaZulu-Natal and Soweto, South Africa; survey period 2009-2011) using an assay that detects 20 ARV drugs (6 nucleoside/nucleotide reverse transcriptase inhibitors, 3 nonnucleoside reverse transcriptase inhibitors, and 9 protease inhibitors; maraviroc; raltegravir).
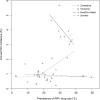
Results: ARV drugs were detected in 2011 (27.4%) of 7347 samples; 88.1% had 1 nonnucleoside reverse transcriptase inhibitors ± 1-2 nucleoside/nucleotide reverse transcriptase inhibitors. ARV drug detection was associated with sex (women>men), pregnancy, older age (>24 years), and study site (P < 0.0001 for all 4 variables). ARV drugs were also more frequently detected in adults who were widowed (P = 0.006) or unemployed (P = 0.02). ARV drug use was more frequent in intervention versus control communities early in the survey (P = 0.01), with a significant increase in control (P = 0.004) but not in intervention communities during the survey period. In KwaZulu-Natal, a 1% increase in ARV drug use was associated with a 0.14% absolute decrease in HIV incidence (P = 0.018).
Conclusions: This study used an objective, biomedical approach to assess ARV drug use on a population level. This analysis identified factors associated with ARV drug use and provided information on ARV drug use over time. ARV drug use was associated with lower HIV incidence at 1 study site.
Conflict of interest statement
Conflict of Interests None of the authors has a conflict of interest or potential conflict of interest, with the following exception: William Clarke is a consultant to Thermo Fisher Scientific.
Figures

References
-
- Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. - PubMed
-
- Cohen MS, Chen YQ, McCauley M, et al. Final results of the HPTN 052 randomized controlled trial: antiretroviral therapy prevents HIV transmission [MOAC0101LB]. Presented at: 8th IAS Conference on HIV Pathogenesis, Treatment & Prevention; 2015; Vancouver, Canada.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

