Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs
- PMID: 27828940
- PMCID: PMC5807003
- DOI: 10.1038/nature20138
Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs
Abstract
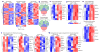
Recognition and removal of apoptotic cells by professional phagocytes, including dendritic cells and macrophages, preserves immune self-tolerance and prevents chronic inflammation and autoimmune pathologies. The diverse array of phagocytes that reside within different tissues, combined with the necessarily prompt nature of apoptotic cell clearance, makes it difficult to study this process in situ. The full spectrum of functions executed by tissue-resident phagocytes in response to homeostatic apoptosis, therefore, remains unclear. Here we show that mouse apoptotic intestinal epithelial cells (IECs), which undergo continuous renewal to maintain optimal barrier and absorptive functions, are not merely extruded to maintain homeostatic cell numbers, but are also sampled by a single subset of dendritic cells and two macrophage subsets within a well-characterized network of phagocytes in the small intestinal lamina propria. Characterization of the transcriptome within each subset before and after in situ sampling of apoptotic IECs revealed gene expression signatures unique to each phagocyte, including macrophage-specific lipid metabolism and amino acid catabolism, and a dendritic-cell-specific program of regulatory CD4+ T-cell activation. A common 'suppression of inflammation' signature was noted, although the specific genes and pathways involved varied amongst dendritic cells and macrophages, reflecting specialized functions. Apoptotic IECs were trafficked to mesenteric lymph nodes exclusively by the dendritic cell subset and served as critical determinants for the induction of tolerogenic regulatory CD4+ T-cell differentiation. Several of the genes that were differentially expressed by phagocytes bearing apoptotic IECs overlapped with susceptibility genes for inflammatory bowel disease. Collectively, these findings provide new insights into the consequences of apoptotic cell sampling, advance our understanding of how homeostasis is maintained within the mucosa and set the stage for development of novel therapeutics to alleviate chronic inflammatory diseases such as inflammatory bowel disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






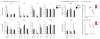



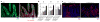


Comment in
-
Gut check: dead cell samples leads to tolerant examples.Cell Death Differ. 2017 Sep;24(9):1471-1472. doi: 10.1038/cdd.2017.45. Epub 2017 Jun 23. Cell Death Differ. 2017. PMID: 28644438 Free PMC article. No abstract available.
References
-
- Bekiaris V, Persson EK, Agace WW. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev. 2014;260:86–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HD007149/HD/NICHD NIH HHS/United States
- R01 CA161373/CA/NCI NIH HHS/United States
- 5T32DK007792-12/NH/NIH HHS/United States
- U19 AI117873/AI/NIAID NIH HHS/United States
- R01 AI095245/AI/NIAID NIH HHS/United States
- DK072201/NH/NIH HHS/United States
- R01 DK110352/DK/NIDDK NIH HHS/United States
- AI123284/NH/NIH HHS/United States
- R01 DK111862/DK/NIDDK NIH HHS/United States
- HHSN272201000054C/AI/NIAID NIH HHS/United States
- 5R01CA161373-04/NH/NIH HHS/United States
- 5P01DK072201-09/NH/NIH HHS/United States
- T32 DK007792/DK/NIDDK NIH HHS/United States
- U01 DK062429/DK/NIDDK NIH HHS/United States
- U01 DK062422/DK/NIDDK NIH HHS/United States
- P01 DK072201/DK/NIDDK NIH HHS/United States
- AI095245/NH/NIH HHS/United States
- 2T32A1007605-11/NH/NIH HHS/United States
- R01 DK092235/DK/NIDDK NIH HHS/United States
- R01 AI123284/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

