Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour
- PMID: 27828941
- PMCID: PMC5161596
- DOI: 10.1038/nature20145
Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour
Abstract
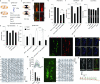
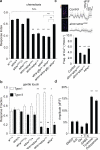
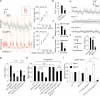
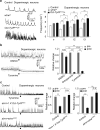
Astrocytes associate with synapses throughout the brain and express receptors for neurotransmitters that can increase intracellular calcium (Ca2+). Astrocytic Ca2+ signalling has been proposed to modulate neural circuit activity, but the pathways that regulate these events are poorly defined and in vivo evidence linking changes in astrocyte Ca2+ levels to alterations in neurotransmission or behaviour is limited. Here we show that Drosophila astrocytes exhibit activity-regulated Ca2+ signalling in vivo. Tyramine and octopamine released from neurons expressing tyrosine decarboxylase 2 (Tdc2) signal directly to astrocytes to stimulate Ca2+ increases through the octopamine/tyramine receptor (Oct-TyrR) and the transient receptor potential (TRP) channel Water witch (Wtrw), and astrocytes in turn modulate downstream dopaminergic neurons. Application of tyramine or octopamine to live preparations silenced dopaminergic neurons and this inhibition required astrocytic Oct-TyrR and Wtrw. Increasing astrocyte Ca2+ signalling was sufficient to silence dopaminergic neuron activity, which was mediated by astrocyte endocytic function and adenosine receptors. Selective disruption of Oct-TyrR or Wtrw expression in astrocytes blocked astrocytic Ca2+ signalling and profoundly altered olfactory-driven chemotaxis and touch-induced startle responses. Our work identifies Oct-TyrR and Wtrw as key components of the astrocytic Ca2+ signalling machinery, provides direct evidence that octopamine- and tyramine-based neuromodulation can be mediated by astrocytes, and demonstrates that astrocytes are essential for multiple sensory-driven behaviours in Drosophila.
Figures

References
-
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. - PubMed
-
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. - PubMed
-
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. - PubMed
-
- Smith SJ. Do astrocytes process neural information? Prog. Brain Res. 1992;94:119–136. - PubMed
Materials and methods references
-
- Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

