Cigarette Smoke Modulates Repair and Innate Immunity following Injury to Airway Epithelial Cells
- PMID: 27829065
- PMCID: PMC5102360
- DOI: 10.1371/journal.pone.0166255
Cigarette Smoke Modulates Repair and Innate Immunity following Injury to Airway Epithelial Cells
Abstract
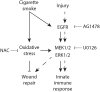
Cigarette smoking is the main risk factor associated with chronic obstructive pulmonary disease (COPD), and contributes to COPD development and progression by causing epithelial injury and inflammation. Whereas it is known that cigarette smoke (CS) may affect the innate immune function of airway epithelial cells and epithelial repair, this has so far not been explored in an integrated design using mucociliary differentiated airway epithelial cells. In this study, we examined the effect of whole CS exposure on wound repair and the innate immune activity of mucociliary differentiated primary bronchial epithelial cells, upon injury induced by disruption of epithelial barrier integrity or by mechanical wounding. Upon mechanical injury CS caused a delayed recovery in the epithelial barrier integrity and wound closure. Furthermore CS enhanced innate immune responses, as demonstrated by increased expression of the antimicrobial protein RNase 7. These differential effects on epithelial repair and innate immunity were both mediated by CS-induced oxidative stress. Overall, our findings demonstrate modulation of wound repair and innate immune responses of injured airway epithelial cells that may contribute to COPD development and progression.
Conflict of interest statement
This research was funded by an unrestricted research grant by Galapagos N.V., the Netherlands. We confirm that this does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Brusselle GG, Joos GF, Bracke KR. New Insights into the Immunology of Chronic Obstructive Pulmonary Disease. The Lancet 2011;378:1015–1026. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

