Unusual reactions of diazocarbonyl compounds with α,β-unsaturated δ-amino esters: Rh(II)-catalyzed Wolff rearrangement and oxidative cleavage of N-H-insertion products
- PMID: 27829897
- PMCID: PMC5082446
- DOI: 10.3762/bjoc.12.180
Unusual reactions of diazocarbonyl compounds with α,β-unsaturated δ-amino esters: Rh(II)-catalyzed Wolff rearrangement and oxidative cleavage of N-H-insertion products
Abstract
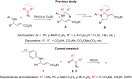
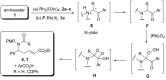
Rh(II)-сatalyzed reactions of aroyldiazomethanes, diazoketoesters and diazodiketones with α,β-unsaturated δ-aminoesters, in contrast to reactions of diazomalonates and other diazoesters, give rise to the Wolff rearrangement and/or oxidative cleavage of the initially formed N-H-insertion products. These oxidation processes are mediated by Rh(II) catalysts possessing perfluorinated ligands. The formation of pyrrolidine structures, characteristic for catalytic reactions of diazoesters, was not observed in these processes at all.
Keywords: N–H-insertion; Wolff rearrangement; diazo compounds; oxidation cleavage; transition-metal-catalyzed reactions.
Figures
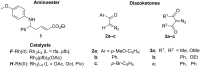
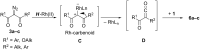
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
