Enantioconvergent catalysis
- PMID: 27829909
- PMCID: PMC5082454
- DOI: 10.3762/bjoc.12.192
Enantioconvergent catalysis
Abstract
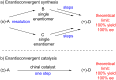
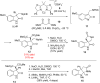
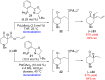
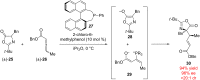
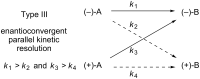
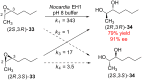
An enantioconvergent catalytic process has the potential to convert a racemic starting material to a single highly enantioenriched product with a maximum yield of 100%. Three mechanistically distinct approaches to effecting enantioconvergent catalysis are identified, and recent examples of each are highlighted. These processes are compared to related, non-enantioconvergent methods.
Keywords: asymmetric catalysis; enantioselectivity; synthetic methods.
Figures
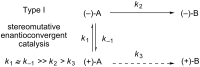


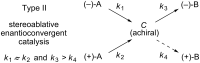


References
-
- Eliel E L, Wilen S H, Mander L N. Stereochemistry of Organic Compounds. New York: Wiley-Interscience; 1994. pp. 963–965. See for a summary of the enantioconvergent synthetic strategy.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
