Intestinal bacterium-derived cyp27a1 prevents colon cancer cell apoptosis
- PMID: 27830027
- PMCID: PMC5095336
Intestinal bacterium-derived cyp27a1 prevents colon cancer cell apoptosis
Abstract
The pathogenesis of metastasis of colon cancer (Cca) is to be further investigated. The dysfunction of apoptotic mechanism plays a role in the cancer cell over growth. This study tests a hypothesis by which intestinal bacterium-derived cyp27a1 prevents apoptosis in colon cancer cells. In this study, the levels of cyp27a1 in human stool samples were assessed by enzyme-linked immunosorbent assay. The apoptosis of Cca cells was observed by flow cytometry. The expression of cyp27a1 was assessed by real time RT-PCR and Western blotting. We observed higher levels of cyp27a1 in the stool samples of Cca patients than that from healthy subjects. Cca colon epithelial biopsy contained high levels of cyp27a1 protein, but not the cyp27a1 mRNA. Cyp27a1 prevented Cca cell apoptosis induced by vitamin D3. In conclusion, intestinal bacterium-derived cyp27a1 facilitates Cca survival by inhibiting Cca cell apoptosis.
Keywords: Colon cancer; Cyp27a1; apoptosis; vitamin D.
Figures
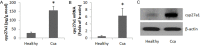



Similar articles
-
Immunoglobulin E induces colon cancer cell apoptosis via enhancing cyp27b1 expression.Am J Transl Res. 2016 Dec 15;8(12):5715-5722. eCollection 2016. Am J Transl Res. 2016. PMID: 28078042 Free PMC article.
-
Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite.Biochem Biophys Res Commun. 2006 Oct 13;349(1):209-13. doi: 10.1016/j.bbrc.2006.08.034. Epub 2006 Aug 15. Biochem Biophys Res Commun. 2006. PMID: 16930540
-
22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma.Cancer. 2010 Dec 1;116(23):5535-43. doi: 10.1002/cncr.25478. Epub 2010 Aug 2. Cancer. 2010. PMID: 20681031
-
Curcumin-mediated regulation of Notch1/hairy and enhancer of split-1/survivin: molecular targeting in cholangiocarcinoma.J Surg Res. 2015 Oct;198(2):434-40. doi: 10.1016/j.jss.2015.03.029. Epub 2015 Mar 19. J Surg Res. 2015. PMID: 25890434 Review.
-
Vitamin D and colon cancer.World J Gastrointest Oncol. 2014 Nov 15;6(11):430-7. doi: 10.4251/wjgo.v6.i11.430. World J Gastrointest Oncol. 2014. PMID: 25400874 Free PMC article. Review.
Cited by
-
Whole Transcriptome Data Analysis Reveals Prognostic Signature Genes for Overall Survival Prediction in Diffuse Large B Cell Lymphoma.Front Genet. 2021 Jun 9;12:648800. doi: 10.3389/fgene.2021.648800. eCollection 2021. Front Genet. 2021. PMID: 34178023 Free PMC article.
-
CYP27A1 inhibits bladder cancer cells proliferation by regulating cholesterol homeostasis.Cell Cycle. 2019 Jan;18(1):34-45. doi: 10.1080/15384101.2018.1558868. Epub 2018 Dec 30. Cell Cycle. 2019. PMID: 30563407 Free PMC article.
References
-
- Adler J, Robertson DJ. Interval Colorectal Cancer After Colonoscopy: exploring Explanations and Solutions. Am J Gastroenterol. 2015;110:1657–1664. - PubMed
-
- Di Sabatino A, Lenti MV, Giuffrida P, Vanoli A, Corazza GR. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun Rev. 2015;14:1161–1169. - PubMed
-
- Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, Rafferty JF Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58:713–725. - PubMed
LinkOut - more resources
Full Text Sources
