Nonvascular retinal imaging markers of preclinical Alzheimer's disease
- PMID: 27830174
- PMCID: PMC5078641
- DOI: 10.1016/j.dadm.2016.09.001
Nonvascular retinal imaging markers of preclinical Alzheimer's disease
Abstract
Introduction: In patients with Alzheimer's disease (AD) and mild cognitive impairment, structural changes in the retina (i.e., reduced thicknesses of the ganglion cell and retinal nerve fiber layers and inclusion bodies that appear to contain beta-amyloid protein [Ab]) have been previously reported. We sought to explore whether anatomic retinal changes are detectable in the preclinical stage of AD.
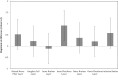
Methods: A cross-sectional study (as part of an ongoing longitudinal cohort study) involving 63 cognitively normal adults, all of whom have a parent with AD and subjective memory complaints. We compared neocortical amyloid aggregation (florbetapir PET imaging) to retinal spectral domain optical coherence tomography (SD-OCT) markers of possible disease burden. Retinal biomarkers, including the number and surface area of retinal inclusion bodies and the thickness of retinal neuronal layers, were compared across groups with high vs. low neocortical beta-amyloid load.
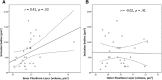
Results: The surface area of inclusion bodies increased as a function of cortical amyloid burden. Additionally, there was a trend toward a selective volume increase in the inner plexiform layer (IPL; a layer rich in cholinergic activity) of the retina in Aβ+ relative to Aβ- participants, and IPL volume was correlated with the surface area of retinal inclusion bodies.
Discussion: These initial results suggest that retinal imaging may be a potential cost-effective and noninvasive technique that can be used to identify those at-risk for AD. Layer-specific changes in the IPL and their association with surface area of inclusion bodies are discussed as a possible reflection of early inflammatory processes associated with cholinergic disruption and concurrent Ab accumulation in the neocortex.
Keywords: Acetylcholine; Alzheimer's disease; Biomarkers; Cholinergic hypothesis; Inner plexiform layer; OCT; Optical Coherence Tomography; Preclinical AD; Retina.
Figures


Similar articles
-
Longitudinal retinal layer changes in preclinical Alzheimer's disease.Acta Ophthalmol. 2021 Aug;99(5):538-544. doi: 10.1111/aos.14640. Epub 2020 Oct 18. Acta Ophthalmol. 2021. PMID: 33073531 Free PMC article.
-
Retinal ganglion cell-inner plexiform layer thickness is nonlinearly associated with cognitive impairment in the community-dwelling elderly.Alzheimers Dement (Amst). 2018 Nov 12;11:19-27. doi: 10.1016/j.dadm.2018.10.006. eCollection 2019 Dec. Alzheimers Dement (Amst). 2018. PMID: 30581972 Free PMC article.
-
Change in retinal structural anatomy during the preclinical stage of Alzheimer's disease.Alzheimers Dement (Amst). 2018 Feb 7;10:196-209. doi: 10.1016/j.dadm.2018.01.003. eCollection 2018. Alzheimers Dement (Amst). 2018. PMID: 29780864 Free PMC article.
-
Optical Coherence Tomography in Alzheimer's Disease and Other Neurodegenerative Diseases.Front Neurol. 2017 Dec 19;8:701. doi: 10.3389/fneur.2017.00701. eCollection 2017. Front Neurol. 2017. PMID: 29312125 Free PMC article. Review.
-
Retinal Vasculopathy in Alzheimer's Disease.Front Neurosci. 2021 Sep 22;15:731614. doi: 10.3389/fnins.2021.731614. eCollection 2021. Front Neurosci. 2021. PMID: 34630020 Free PMC article. Review.
Cited by
-
Identification of early pericyte loss and vascular amyloidosis in Alzheimer's disease retina.Acta Neuropathol. 2020 May;139(5):813-836. doi: 10.1007/s00401-020-02134-w. Epub 2020 Feb 10. Acta Neuropathol. 2020. PMID: 32043162 Free PMC article.
-
The Value of OCT and OCTA as Potential Biomarkers for Preclinical Alzheimer's Disease: A Review Study.Life (Basel). 2021 Jul 19;11(7):712. doi: 10.3390/life11070712. Life (Basel). 2021. PMID: 34357083 Free PMC article. Review.
-
Feasibility study for detection of retinal amyloid in clinical trials: The Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) trial.Alzheimers Dement (Amst). 2021 Aug 17;13(1):e12199. doi: 10.1002/dad2.12199. eCollection 2021. Alzheimers Dement (Amst). 2021. PMID: 34430703 Free PMC article.
-
Retinal Dysfunction in Alzheimer's Disease and Implications for Biomarkers.Biomolecules. 2021 Aug 16;11(8):1215. doi: 10.3390/biom11081215. Biomolecules. 2021. PMID: 34439882 Free PMC article. Review.
-
Anatomical and functional changes in the retina in patients with Alzheimer's disease and mild cognitive impairment.Acta Ophthalmol. 2020 Nov;98(7):e914-e921. doi: 10.1111/aos.14419. Epub 2020 Mar 25. Acta Ophthalmol. 2020. PMID: 32212415 Free PMC article.
References
-
- Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid β deposition, neurodegeneration and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurol. 2013;12:357–367. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
