Functions of Intracellular Retinoid Binding-Proteins
- PMID: 27830500
- PMCID: PMC5493979
- DOI: 10.1007/978-94-024-0945-1_2
Functions of Intracellular Retinoid Binding-Proteins
Abstract
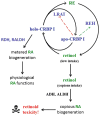
Multiple binding and transport proteins facilitate many aspects of retinoid biology through effects on retinoid transport, cellular uptake, metabolism, and nuclear delivery. These include the serum retinol binding protein sRBP (aka Rbp4), the plasma membrane sRBP receptor Stra6, and the intracellular retinoid binding-proteins such as cellular retinol-binding proteins (CRBP) and cellular retinoic acid binding-proteins (CRABP). sRBP transports the highly lipophilic retinol through an aqueous medium. The major intracellular retinol-binding protein, CRBP1, likely enhances efficient retinoid use by providing a sink to facilitate retinol uptake from sRBP through the plasma membrane or via Stra6, delivering retinol or retinal to select enzymes that generate retinyl esters or retinoic acid, and protecting retinol/retinal from excess catabolism or opportunistic metabolism. Intracellular retinoic acid binding-proteins (CRABP1 and 2, and FABP5) seem to have more diverse functions distinctive to each, such as directing retinoic acid to catabolism, delivering retinoic acid to specific nuclear receptors, and generating non-canonical actions. Gene ablation of intracellular retinoid binding-proteins does not cause embryonic lethality or gross morphological defects. Metabolic and functional defects manifested in knockouts of CRBP1, CRBP2 and CRBP3, however, illustrate their essentiality to health, and in the case of CRBP2, to survival during limited dietary vitamin A. Future studies should continue to address the specific molecular interactions that occur between retinoid binding-proteins and their targets and their precise physiologic contributions to retinoid homeostasis and function.
Keywords: Acyl-CoA:diacylglycerol acyltransferase; Acyl-CoA:monoacylglycerol acyltransferase; Acyl-CoA:retinol acyltransferase; Cellular retinoic acid binding-protein; Cellular retinol binding-protein; Cytochrome P-450; Lecithin:retinol acyltransferase; Peroxisomal proliferator activated receptor δ/β; Retinal; Retinal dehydrogenase; Retinoic acid; Retinoic acid receptor; Retinol; Retinol dehydrogenase; Retinyl ester hydrolase; Serum retinol binding-protein.
Figures






References
-
- Ahn T, Kim M, Yun C-H, Chae H-J. Functional regulation of hepatic cytochrome p450 enzymes by physicochemical properties of phospholipids in biological membranes. Curr Protein Pept Sci. 2007;8:496–505. - PubMed
-
- Akerstrom B, Flower DR, Salier JP. Lipocalins: unity in diversity. Biochim Biophys Acta. 2000;1482:1–8. - PubMed
-
- Bashor MM, Chytil F. Cellular retinol-binding protein. Biochim Biophys Acta. 1975;411:87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

