The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail
- PMID: 27831554
- PMCID: PMC5260884
- DOI: 10.1038/cddis.2016.344
The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail
Abstract
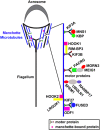
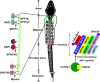
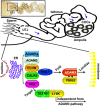
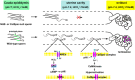
Male infertility due to abnormal spermatozoa has been reported in both animals and humans, but its pathogenic causes, including genetic abnormalities, remain largely unknown. On the other hand, contraceptive options for men are limited, and a specific, reversible and safe method of male contraception has been a long-standing quest in medicine. Some progress has recently been made in exploring the effects of spermatid-specifical genetic factors in controlling male fertility. A comprehensive search of PubMed for articles and reviews published in English before July 2016 was carried out using the search terms 'spermiogenesis failure', 'globozoospermia', 'spermatid-specific', 'acrosome', 'infertile', 'manchette', 'sperm connecting piece', 'sperm annulus', 'sperm ADAMs', 'flagellar abnormalities', 'sperm motility loss', 'sperm ion exchanger' and 'contraceptive targets'. Importantly, we have opted to focus on articles regarding spermatid-specific factors. Genetic studies to define the structure and physiology of sperm have shown that spermatozoa appear to be one of the most promising contraceptive targets. Here we summarize how these spermatid-specific factors regulate spermiogenesis and categorize them according to their localization and function from spermatid head to tail (e.g., acrosome, manchette, head-tail conjunction, annulus, principal piece of tail). In addition, we emphatically introduce small-molecule contraceptives, such as BRDT and PPP3CC/PPP3R2, which are currently being developed to target spermatogenic-specific proteins. We suggest that blocking the differentiation of haploid germ cells, which rarely affects early spermatogenic cell types and the testicular microenvironment, is a better choice than spermatogenic-specific proteins. The studies described here provide valuable information regarding the genetic and molecular defects causing male mouse infertility to improve our understanding of the importance of spermatid-specific factors in controlling fertility. Although a male contraceptive 'pill' is still many years away, research into the production of new small-molecule contraceptives targeting spermatid-specific proteins is the right avenue.
Figures

References
-
- Skakkebaek NE, Jorgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM et al. Is human fecundity declining? Int J Androl 2006; 29: 2–11. - PubMed
-
- Liu PY, Handelsman DJ. The present and future state of hormonal treatment for male infertility. Hum Reprod Update 2003; 9: 9–23. - PubMed
-
- Georgiou I, Syrrou M, Pardalidis N, Karakitsios K, Mantzavinos T, Giotitsas N et al. Genetic and epigenetic risks of intracytoplasmic sperm injection method. Asian J Androl 2006; 8: 643–673. - PubMed
-
- O'Flynn O'Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril 2010; 93: 1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

