Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage
- PMID: 27831562
- PMCID: PMC5260876
- DOI: 10.1038/cddis.2016.358
Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage
Abstract
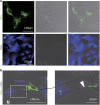
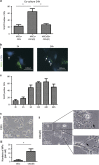
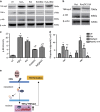
Recent studies have demonstrated that mesenchymal stem cells (MSCs) can donate mitochondria to airway epithelial cells and rescue mitochondrial damage in lung injury. We sought to determine whether MSCs could donate mitochondria and protect against oxidative stress-induced mitochondrial dysfunction in the cornea. Co-culturing of MSCs and corneal epithelial cells (CECs) indicated that the efficiency of mitochondrial transfer from MSCs to CECs was enhanced by Rotenone (Rot)-induced oxidative stress. The efficient mitochondrial transfer was associated with increased formation of tunneling nanotubes (TNTs) between MSCs and CECs, tubular connections that allowed direct intercellular communication. Separation of MSCs and CECs by a transwell culture system revealed no mitochiondrial transfer from MSCs to CECs and mitochondrial function was impaired when CECs were exposed to Rot challenge. CECs with or without mitochondrial transfer from MSCs displayed a distinct survival capacity and mitochondrial oxygen consumption rate. Mechanistically, increased filopodia outgrowth in CECs for TNT formation was associated with oxidative inflammation-activated NFκB/TNFαip2 signaling pathways that could be attenuated by reactive oxygen species scavenger N-acetylcysteine (NAC) treatment. Furthermore, MSCs grown on a decellularized porcine corneal scaffold were transplanted onto an alkali-injured eye in a rabbit model. Enhanced corneal wound healing was evident following healthy MSC scaffold transplantation. And transferred mitochondria was detected in corneal epithelium. In conclusion, mitochondrial transfer from MSCs provides novel protection for the cornea against oxidative stress-induced mitochondrial damage. This therapeutic strategy may prove relevant for a broad range of mitochondrial diseases.
Figures


Similar articles
-
Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung.Stem Cell Res Ther. 2016 Jul 12;7(1):91. doi: 10.1186/s13287-016-0354-8. Stem Cell Res Ther. 2016. PMID: 27406134 Free PMC article.
-
Bioenergetic Crosstalk between Mesenchymal Stem Cells and various Ocular Cells through the intercellular trafficking of Mitochondria.Theranostics. 2020 Jun 5;10(16):7260-7272. doi: 10.7150/thno.46332. eCollection 2020. Theranostics. 2020. PMID: 32641991 Free PMC article.
-
Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface.Exp Eye Res. 2013 Nov;116:312-23. doi: 10.1016/j.exer.2013.10.002. Epub 2013 Oct 18. Exp Eye Res. 2013. PMID: 24145108
-
Tunneling Nanotubes-Mediated Protection of Mesenchymal Stem Cells: An Update from Preclinical Studies.Int J Mol Sci. 2020 May 14;21(10):3481. doi: 10.3390/ijms21103481. Int J Mol Sci. 2020. PMID: 32423160 Free PMC article. Review.
-
Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer.Semin Cell Dev Biol. 2016 Apr;52:119-31. doi: 10.1016/j.semcdb.2016.02.011. Epub 2016 Feb 8. Semin Cell Dev Biol. 2016. PMID: 26868759 Review.
Cited by
-
Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation.Stem Cell Reports. 2018 Nov 13;11(5):1120-1135. doi: 10.1016/j.stemcr.2018.09.012. Epub 2018 Oct 18. Stem Cell Reports. 2018. PMID: 30344008 Free PMC article.
-
Mitochondrial transplantation as a potential and novel master key for treatment of various incurable diseases.Cytotechnology. 2019 Apr;71(2):647-663. doi: 10.1007/s10616-019-00302-9. Epub 2019 Jan 31. Cytotechnology. 2019. PMID: 30706303 Free PMC article. Review.
-
Rhes Tunnels: A Radical New Way of Communication in the Brain's Striatum?Bioessays. 2020 Jun;42(6):e1900231. doi: 10.1002/bies.201900231. Epub 2020 Apr 1. Bioessays. 2020. PMID: 32236969 Free PMC article. Review.
-
Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function.Sci Rep. 2018 Feb 20;8(1):3330. doi: 10.1038/s41598-018-21539-y. Sci Rep. 2018. PMID: 29463809 Free PMC article.
-
Paracrine activity of adipose derived stem cells on limbal epithelial stem cells.Sci Rep. 2021 Oct 7;11(1):19956. doi: 10.1038/s41598-021-99435-1. Sci Rep. 2021. PMID: 34620960 Free PMC article.
References
-
- Cejka C, Holan V, Trosan P, Zajicova A, Javorkova E, Cejkova J. The favorable effect of mesenchymal stem cell treatment on the antioxidant protective mechanism in the corneal epithelium and renewal of corneal optical properties changed after alkali burns. Oxid Med Cell Longev 2016; 25: 874–881. - PMC - PubMed
-
- Shi X, Zhou B. The role of nrf2 and mapk pathways in pfos-induced oxidative stress in zebrafish embryos. Toxicol Sci 2010; 115: 391–400. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

