Silencing of CDK2, but not CDK1, separates mitogenic from anti-apoptotic signaling, sensitizing p53 defective cells for synthetic lethality
- PMID: 27831832
- PMCID: PMC5176141
- DOI: 10.1080/15384101.2016.1241915
Silencing of CDK2, but not CDK1, separates mitogenic from anti-apoptotic signaling, sensitizing p53 defective cells for synthetic lethality
Abstract
Small molecule inhibitors targeting CDK1/CDK2 have been clinically proven effective against a variety of tumors, albeit at the cost of profound off target toxicities. To separate potential therapeutic from toxic effects, we selectively knocked down CDK1 or CDK2 in p53 mutated HACAT cells by siRNA silencing. Using dynamic, cell cycle wide proteome arrays, we observed minor changes in overall abundance of proteins critically involved in cell cycle transition despite profound G2/M or G1/S arrest, respectively. Employing phospho site specific analyses, we identified uncoupled mitogenic, yet pro-apoptotic signaling from counter balancing anti-apoptotic activity in CDK2 disrupted cells. Moreover, a crucial role of CDK2 activity in early serum response was observed, extending well-established roles of CDKs outside their cell cycle regulating functions. In contrast, disruption of CDK1 only marginally affected phosphorylation events of crucial signaling nodes prior to G2/S transition. The data presented here suggest that the temporal separation of pro- and anti-apoptotic pathways by selective inhibition of CDK2 disrupts coherent signaling modules and may synergize with anti-proliferative drugs, averting toxic side effects from CDK1 inhibition.
Keywords: CDK1; CDK2; HACAT; cell cycle; p53; phosphorylation; serine-threonine kinase; signaling pathway; tyrosine kinase.
Figures
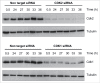
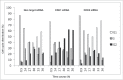

Similar articles
-
Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells.BMC Cancer. 2014 Jan 20;14:32. doi: 10.1186/1471-2407-14-32. BMC Cancer. 2014. PMID: 24444383 Free PMC article.
-
Silencing of Dicer1 temporally separates pro- and anti-apoptotic signaling and confers susceptibility to chemotherapy in p53 mutated cells.Cell Cycle. 2014;13(14):2192-8. doi: 10.4161/cc.29216. Epub 2014 May 20. Cell Cycle. 2014. PMID: 24846461 Free PMC article.
-
New alternative phosphorylation sites on the cyclin dependent kinase 1/cyclin a complex in p53-deficient human cells treated with etoposide: possible association with etoposide-induced apoptosis.Apoptosis. 2007 Oct;12(10):1847-55. doi: 10.1007/s10495-007-0104-6. Apoptosis. 2007. PMID: 17636382
-
Critical reanalysis of the methods that discriminate the activity of CDK2 from CDK1.Cell Cycle. 2016 May 2;15(9):1184-8. doi: 10.1080/15384101.2016.1160983. Epub 2016 Mar 17. Cell Cycle. 2016. PMID: 26986210 Free PMC article. Review.
-
Chemical and biological profile of dual Cdk1 and Cdk2 inhibitors.Curr Med Chem Anticancer Agents. 2003 Jan;3(1):1-14. doi: 10.2174/1568011033353605. Curr Med Chem Anticancer Agents. 2003. PMID: 12678910 Review.
Cited by
-
Synthetic Lethality-based Identification of Targets for Anticancer Drugs in the Human Signaling Network.Sci Rep. 2018 May 31;8(1):8440. doi: 10.1038/s41598-018-26783-w. Sci Rep. 2018. PMID: 29855504 Free PMC article.
-
Cyclin-dependent kinases-based synthetic lethality: Evidence, concept, and strategy.Acta Pharm Sin B. 2021 Sep;11(9):2738-2748. doi: 10.1016/j.apsb.2021.01.002. Epub 2021 Jan 7. Acta Pharm Sin B. 2021. PMID: 34589394 Free PMC article. Review.
-
Cyclin-Dependent Kinase Synthetic Lethality Partners in DNA Damage Response.Int J Mol Sci. 2022 Mar 24;23(7):3555. doi: 10.3390/ijms23073555. Int J Mol Sci. 2022. PMID: 35408915 Free PMC article. Review.
-
Identification of Potential Key Genes and Prognostic Biomarkers of Lung Cancer Based on Bioinformatics.Biomed Res Int. 2023 Jan 18;2023:2152432. doi: 10.1155/2023/2152432. eCollection 2023. Biomed Res Int. 2023. PMID: 36714024 Free PMC article.
-
Comprehensive analysis of differential expression profiles reveals potential biomarkers associated with the cell cycle and regulated by p53 in human small cell lung cancer.Exp Ther Med. 2018 Apr;15(4):3273-3282. doi: 10.3892/etm.2018.5833. Epub 2018 Feb 2. Exp Ther Med. 2018. PMID: 29545845 Free PMC article.
References
-
- Canavese M, Santo L, Raje N. Cyclin dependent kinases in cancer: potential for therapeutic intervention. Cancer Biol Ther 2012; 13(7):451-7; PMID:22361734; http://dx.doi.org/10.4161/cbt.19589 - DOI - PubMed
-
- Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, Zamboni WC, Wong KK, Perou CM, Sharpless NE. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst 2012; 104(6):476-87; PMID:22302033; http://dx.doi.org/10.1093/jnci/djs002 - DOI - PMC - PubMed
-
- Kumar SK, LaPlant B, Chng WJ, Zonder J, Callander N, Fonseca R, Fruth B, Roy V, Erlichman C, Stewart AK. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015; 125(3):443-8; PMID:25395429; http://dx.doi.org/10.1182/blood-2014-05-573741 - DOI - PMC - PubMed
-
- Criscitiello C, Viale G, Esposito A, Curigliano G. Dinaciclib for the treatment of breast cancer. Expert Opin Investig Drugs 2014; 23(9):1305-12; PMID:25107301; http://dx.doi.org/10.1517/13543784.2014.948152 - DOI - PubMed
-
- Malumbres M. Cyclin-dependent kinases. Genome Biol 2014; 15(6):122; PMID:25180339; http://dx.doi.org/10.1186/gb4184 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
