Performance of Streck cfDNA Blood Collection Tubes for Liquid Biopsy Testing
- PMID: 27832189
- PMCID: PMC5104415
- DOI: 10.1371/journal.pone.0166354
Performance of Streck cfDNA Blood Collection Tubes for Liquid Biopsy Testing
Abstract
Objectives: Making liquid biopsy testing widely available requires a concept to ship whole blood at ambient temperatures while retaining the integrity of the cell-free DNA (cfDNA) population and stability of blood cells to prevent dilution of circulating tumor DNA (ctDNA) with wild-type genomic DNA. The cell- and DNA-stabilizing properties of Streck Cell-Free DNA BCT blood collection tubes (cfDNA BCTs) were evaluated to determine if they can be utilized in combination with highly sensitive mutation detection technologies.
Methods: Venous blood from healthy donors or patients with advanced colorectal cancer (CRC) was collected in cfDNA BCTs and standard K2EDTA tubes. Tubes were stored at different temperatures for various times before plasma preparation and DNA extraction. The isolated cfDNA was analyzed for overall DNA yield of short and long DNA fragments using qPCR as well as for mutational changes using BEAMing and Plasma Safe-Sequencing (Safe-SeqS).
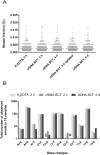
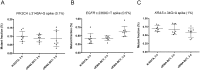
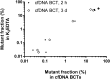
Results: Collection of whole blood from healthy individuals in cfDNA BCTs and storage for up to 5 days at room temperature did not affect the DNA yield and mutation background levels (n = 60). Low-frequency mutant DNA spiked into normal blood samples as well as mutant circulating tumor DNA in blood samples from CRC patients collected in cfDNA BCTs were reliably detected after 3 days of storage at room temperature. However, blood samples stored at ≤ 10°C and at 40°C for an extended period of time showed elevated normal genomic DNA levels and an abnormally large cellular plasma interface as well as lower plasma volumes.
Conclusion: Whole blood shipped in cfDNA BCTs over several days can be used for downstream liquid biopsy testing using BEAMing and Safe-SeqS. Since the shipping temperature is a critical factor, special care has to be taken to maintain a defined room temperature range to obtain reliable mutation testing results.
Conflict of interest statement
All authors are employees of Sysmex Inostics GmbH, whose company funded this study. FD is an inventor on patents related to the BEAMing technology (patents US8715934 B2, “Single-molecule PCR on microparticles in water-in-oil emulsions”; US9360526 B2, “Methods for BEAMing”; and US9360526 B2, “Improved methods for BEAMing”). The Safe-SeqS technology is in development at Sysmex Inostics. OncoBEAM is a product of Sysmex Inostics and is based on the BEAMing technology. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures



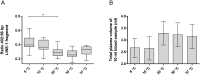
References
-
- Sherwood JL, Corcoran C, Brown H, Sharpe AD, Musilova M, Kohlmann A. Optimised Pre-Analytical Methods Improve KRAS Mutation Detection in Circulating Tumour DNA (ctDNA) from Patients with Non-Small Cell Lung Cancer (NSCLC). PLoS One. 2016;11: e0150197 10.1371/journal.pone.0150197 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

