Evolution of Fitness Cost-Neutral Mutant PfCRT Conferring P. falciparum 4-Aminoquinoline Drug Resistance Is Accompanied by Altered Parasite Metabolism and Digestive Vacuole Physiology
- PMID: 27832198
- PMCID: PMC5104409
- DOI: 10.1371/journal.ppat.1005976
Evolution of Fitness Cost-Neutral Mutant PfCRT Conferring P. falciparum 4-Aminoquinoline Drug Resistance Is Accompanied by Altered Parasite Metabolism and Digestive Vacuole Physiology
Abstract
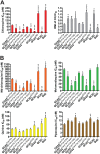
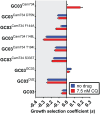
Southeast Asia is an epicenter of multidrug-resistant Plasmodium falciparum strains. Selective pressures on the subcontinent have recurrently produced several allelic variants of parasite drug resistance genes, including the P. falciparum chloroquine resistance transporter (pfcrt). Despite significant reductions in the deployment of the 4-aminoquinoline drug chloroquine (CQ), which selected for the mutant pfcrt alleles that halted CQ efficacy decades ago, the parasite pfcrt locus is continuously evolving. This is highlighted by the presence of a highly mutated allele, Cam734 pfcrt, which has acquired the singular ability to confer parasite CQ resistance without an associated fitness cost. Here, we used pfcrt-specific zinc-finger nucleases to genetically dissect this allele in the pathogenic setting of asexual blood-stage infection. Comparative analysis of drug resistance and growth profiles of recombinant parasites that express Cam734 or variants thereof, Dd2 (the most common Southeast Asian variant), or wild-type pfcrt, revealed previously unknown roles for PfCRT mutations in modulating parasite susceptibility to multiple antimalarial agents. These results were generated in the GC03 strain, used in multiple earlier pfcrt studies, and might differ in natural isolates harboring this allele. Results presented herein show that Cam734-mediated CQ resistance is dependent on the rare A144F mutation that has not been observed beyond Southeast Asia, and reveal distinct impacts of this and other Cam734-specific mutations on CQ resistance and parasite growth rates. Biochemical assays revealed a broad impact of mutant PfCRT isoforms on parasite metabolism, including nucleoside triphosphate levels, hemoglobin catabolism and disposition of heme, as well as digestive vacuole volume and pH. Results from our study provide new insights into the complex molecular basis and physiological impact of PfCRT-mediated antimalarial drug resistance, and inform ongoing efforts to characterize novel pfcrt alleles that can undermine the efficacy of first-line antimalarial drug regimens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


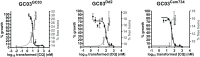

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

