Glycogen synthase kinase-3β regulates fractalkine production by altering its trafficking from Golgi to plasma membrane: implications for Alzheimer's disease
- PMID: 27832289
- PMCID: PMC5309299
- DOI: 10.1007/s00018-016-2408-6
Glycogen synthase kinase-3β regulates fractalkine production by altering its trafficking from Golgi to plasma membrane: implications for Alzheimer's disease
Abstract
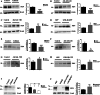
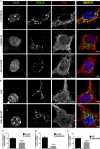
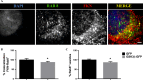
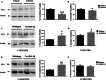
Glycogen synthase kinase-3β (GSK-3β) is a serine-threonine kinase implicated in multiple processes and signaling pathways. Its dysregulation is associated with different pathological conditions including Alzheimer's disease (AD). Here we demonstrate how changes in GSK-3β activity and/or levels regulate the production and subsequent secretion of fractalkine, a chemokine involved in the immune response that has been linked to AD and to other different neurological disorders. Treatment of primary cultured neurons with GSK-3β inhibitors such as lithium and AR-A014418 decreased full-length fractalkine in total cell extracts. Opposite effects were observed after neuron transduction with a lentiviral vector overexpressing the kinase. Biotinylation assays showed that those changes mainly affect the plasma membrane-associated form of the protein, an observation that positively correlates with changes in the levels of its soluble form. These effects were confirmed in lithium-treated wild type (wt) mice and in GSK-3β transgenic animals, as well as in brain samples from AD patients, evident as age-dependent (animals) or Braak stage dependent changes (humans) in both the membrane-bound and the soluble forms of the protein. Further immunohistochemical analyses demonstrated how GSK-3β exerts these effects by affecting the trafficking of the chemokine from the Golgi to the plasma membrane, in different and opposite ways when the levels/activity of the kinase are increased or decreased. This work provides for the first time a mechanism linking GSK-3β and fractalkine both in vitro and in vivo, with important implications for neurological disorders and especially for AD, in which levels of this chemokine might be useful as a diagnostic tool.
Keywords: Alzheimer’s disease; Fractalkine; GSK-3β; Golgi network; Rab8.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Therapeutic implications of glycogen synthase kinase-3β in Alzheimer's disease: a novel therapeutic target.Int J Neurosci. 2024 Jun;134(6):603-619. doi: 10.1080/00207454.2022.2130297. Epub 2022 Oct 10. Int J Neurosci. 2024. PMID: 36178363 Review.
-
RNA Interference Silencing of Glycogen Synthase Kinase 3β Inhibites Tau Phosphorylation in Mice with Alzheimer Disease.Neurochem Res. 2016 Sep;41(9):2470-80. doi: 10.1007/s11064-016-1960-7. Epub 2016 Jun 2. Neurochem Res. 2016. PMID: 27255602
-
Design, Structure-Activity Relationships, and In Vivo Evaluation of Potent and Brain-Penetrant Imidazo[1,2-b]pyridazines as Glycogen Synthase Kinase-3β (GSK-3β) Inhibitors.J Med Chem. 2023 Mar 23;66(6):4231-4252. doi: 10.1021/acs.jmedchem.3c00133. Epub 2023 Mar 9. J Med Chem. 2023. PMID: 36950863
-
Truncation and activation of GSK-3β by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer's disease.Sci Rep. 2015 Feb 2;5:8187. doi: 10.1038/srep08187. Sci Rep. 2015. PMID: 25641096 Free PMC article.
-
Impact of GSK-3β and CK-1δ on Wnt signaling pathway in alzheimer disease: A dual target approach.Bioorg Chem. 2024 Jun;147:107378. doi: 10.1016/j.bioorg.2024.107378. Epub 2024 Apr 17. Bioorg Chem. 2024. PMID: 38643562 Review.
Cited by
-
The Role of Alcohol-Induced Golgi Fragmentation for Androgen Receptor Signaling in Prostate Cancer.Mol Cancer Res. 2019 Jan;17(1):225-237. doi: 10.1158/1541-7786.MCR-18-0577. Epub 2018 Sep 17. Mol Cancer Res. 2019. PMID: 30224543 Free PMC article.
-
Microglia in Alzheimer's Disease in the Context of Tau Pathology.Biomolecules. 2020 Oct 14;10(10):1439. doi: 10.3390/biom10101439. Biomolecules. 2020. PMID: 33066368 Free PMC article. Review.
-
Molecular docking studies and molecular dynamic simulation analysis: To identify novel ATP-competitive inhibition of Glycogen synthase kinase-3β for Alzheimer's disease.F1000Res. 2025 May 27;13:773. doi: 10.12688/f1000research.145391.3. eCollection 2024. F1000Res. 2025. PMID: 40443428 Free PMC article.
-
Anti-oxidant activity of coenzyme Q10 against AlCl3/D-galactose in albino rat induced cognitive dysfunctions: Behavioral, biochemical, and BACE-1/GSK-3β alterations.Toxicol Res (Camb). 2024 Aug 19;13(4):tfae131. doi: 10.1093/toxres/tfae131. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 39165833 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

